| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1391970 | 983689 | 2015 | 12 صفحه PDF | دانلود رایگان |
• A cleavage-coupled macrocyclization activity makes the cyclic seed peptide SFTI-1
• Inefficient macrocyclization is masked by a stepwise breakdown of acyclic products
• First production of asparaginyl endopeptidase (AEP) in E. coli
• Macrocyclization can be reconstituted in vitro, offering insights into biosynthesis
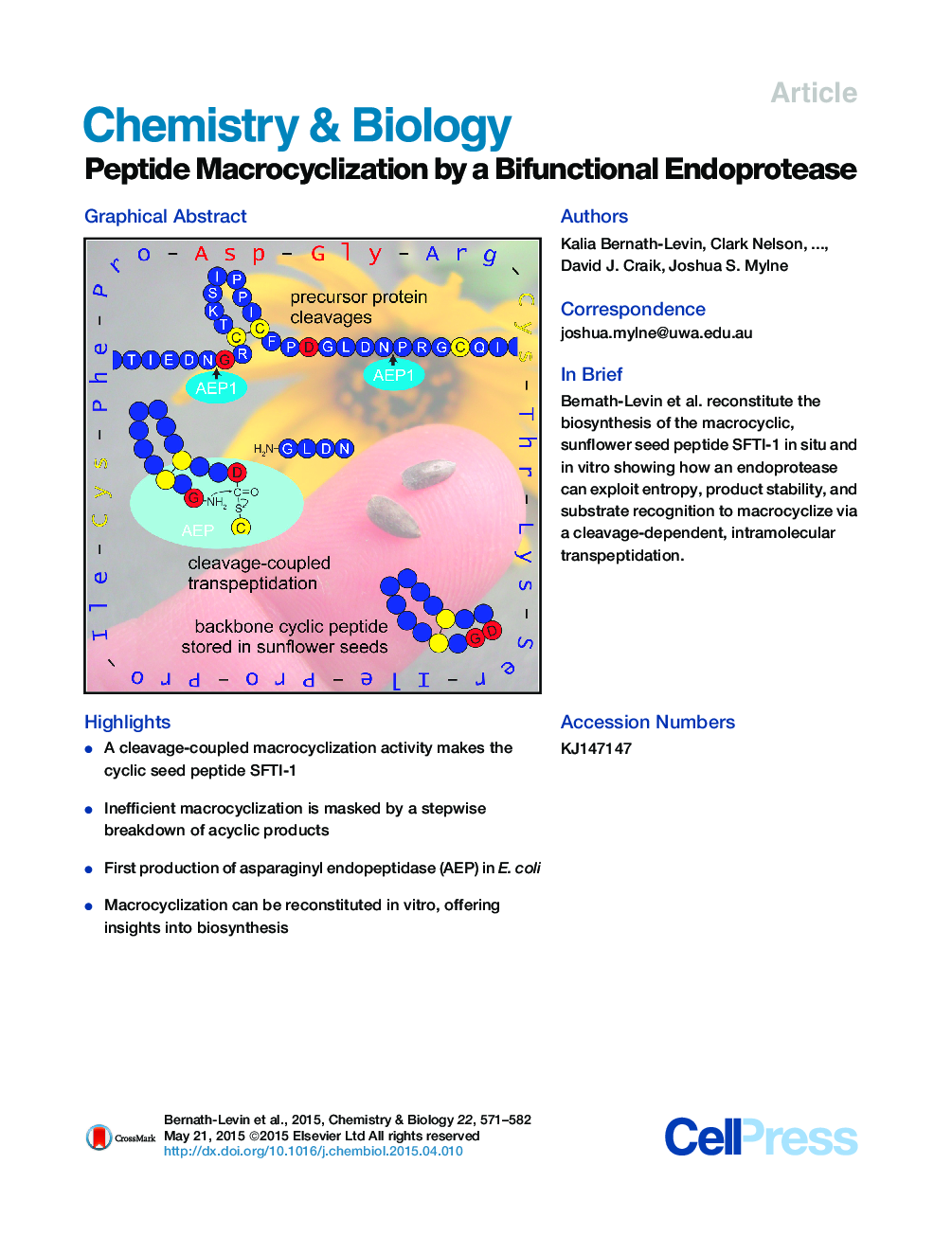
SummaryProteases usually cleave peptides, but under some conditions, they can ligate them. Seeds of the common sunflower contain the 14-residue, backbone-macrocyclic peptide sunflower trypsin inhibitor 1 (SFTI-1) whose maturation from its precursor has a genetic requirement for asparaginyl endopeptidase (AEP). To provide more direct evidence, we developed an in situ assay and used 18O-water to demonstrate that SFTI-1 is excised and simultaneously macrocyclized from its linear precursor. The reaction is inefficient in situ, but a newfound breakdown pathway can mask this inefficiency by reducing the internal disulfide bridge of any acyclic-SFTI to thiols before degrading it. To confirm AEP can directly perform the excision/ligation, we produced several recombinant plant AEPs in E. coli, and one from jack bean could catalyze both a typical cleavage reaction and cleavage-dependent, intramolecular transpeptidation to create SFTI-1. We propose that the evolution of ligating endoproteases enables plants like sunflower and jack bean to stabilize bioactive peptides.
Graphical AbstractFigure optionsDownload high-quality image (286 K)Download as PowerPoint slide
Journal: - Volume 22, Issue 5, 21 May 2015, Pages 571–582