| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145829 | 456352 | 2016 | 11 صفحه PDF | دانلود رایگان |
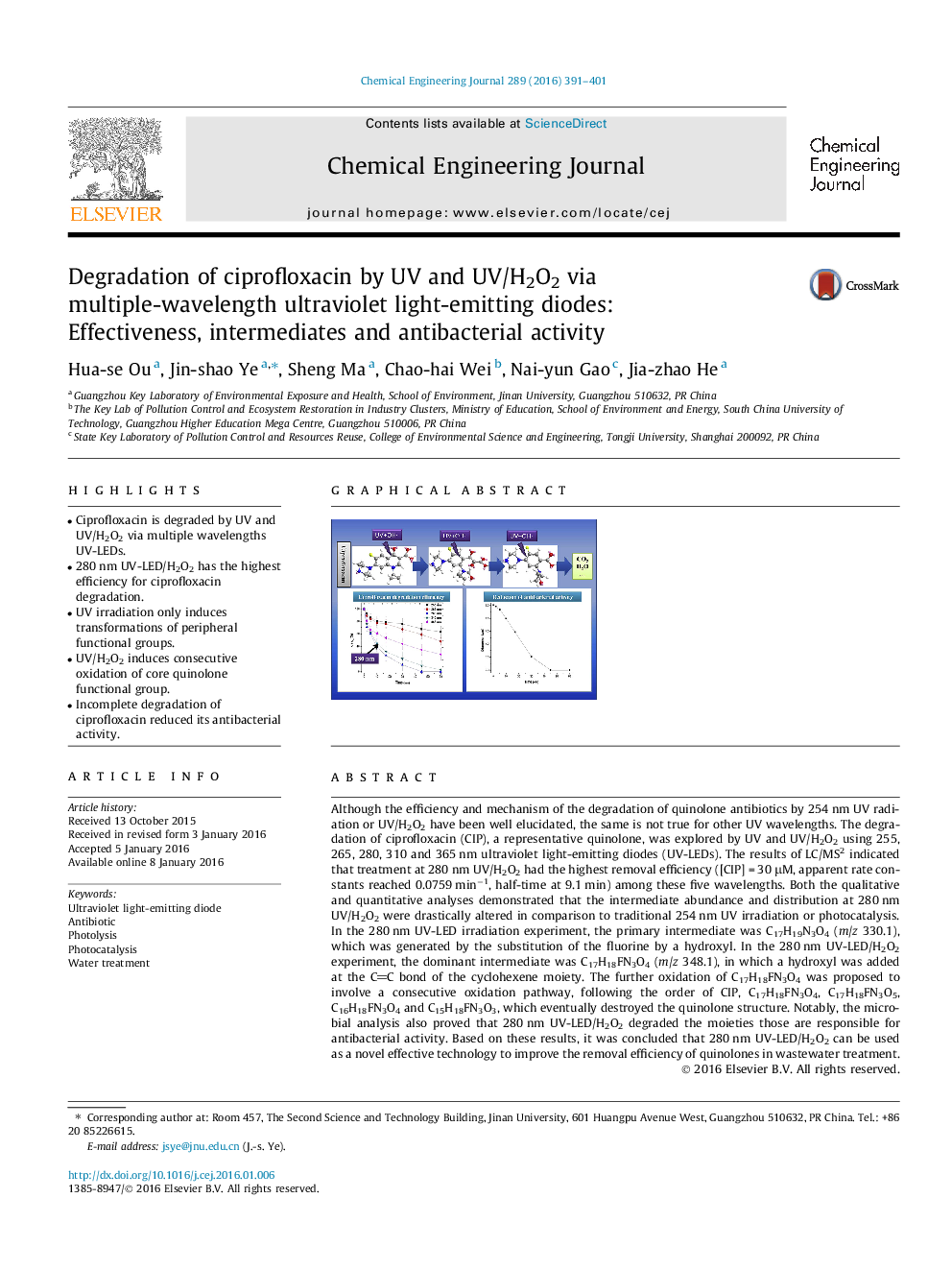
• Ciprofloxacin is degraded by UV and UV/H2O2 via multiple wavelengths UV-LEDs.
• 280 nm UV-LED/H2O2 has the highest efficiency for ciprofloxacin degradation.
• UV irradiation only induces transformations of peripheral functional groups.
• UV/H2O2 induces consecutive oxidation of core quinolone functional group.
• Incomplete degradation of ciprofloxacin reduced its antibacterial activity.
Although the efficiency and mechanism of the degradation of quinolone antibiotics by 254 nm UV radiation or UV/H2O2 have been well elucidated, the same is not true for other UV wavelengths. The degradation of ciprofloxacin (CIP), a representative quinolone, was explored by UV and UV/H2O2 using 255, 265, 280, 310 and 365 nm ultraviolet light-emitting diodes (UV-LEDs). The results of LC/MS2 indicated that treatment at 280 nm UV/H2O2 had the highest removal efficiency ([CIP] = 30 μM, apparent rate constants reached 0.0759 min−1, half-time at 9.1 min) among these five wavelengths. Both the qualitative and quantitative analyses demonstrated that the intermediate abundance and distribution at 280 nm UV/H2O2 were drastically altered in comparison to traditional 254 nm UV irradiation or photocatalysis. In the 280 nm UV-LED irradiation experiment, the primary intermediate was C17H19N3O4 (m/z 330.1), which was generated by the substitution of the fluorine by a hydroxyl. In the 280 nm UV-LED/H2O2 experiment, the dominant intermediate was C17H18FN3O4 (m/z 348.1), in which a hydroxyl was added at the CC bond of the cyclohexene moiety. The further oxidation of C17H18FN3O4 was proposed to involve a consecutive oxidation pathway, following the order of CIP, C17H18FN3O4, C17H18FN3O5, C16H18FN3O4 and C15H18FN3O3, which eventually destroyed the quinolone structure. Notably, the microbial analysis also proved that 280 nm UV-LED/H2O2 degraded the moieties those are responsible for antibacterial activity. Based on these results, it was concluded that 280 nm UV-LED/H2O2 can be used as a novel effective technology to improve the removal efficiency of quinolones in wastewater treatment.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 289, 1 April 2016, Pages 391–401