| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148453 | 456416 | 2013 | 11 صفحه PDF | دانلود رایگان |
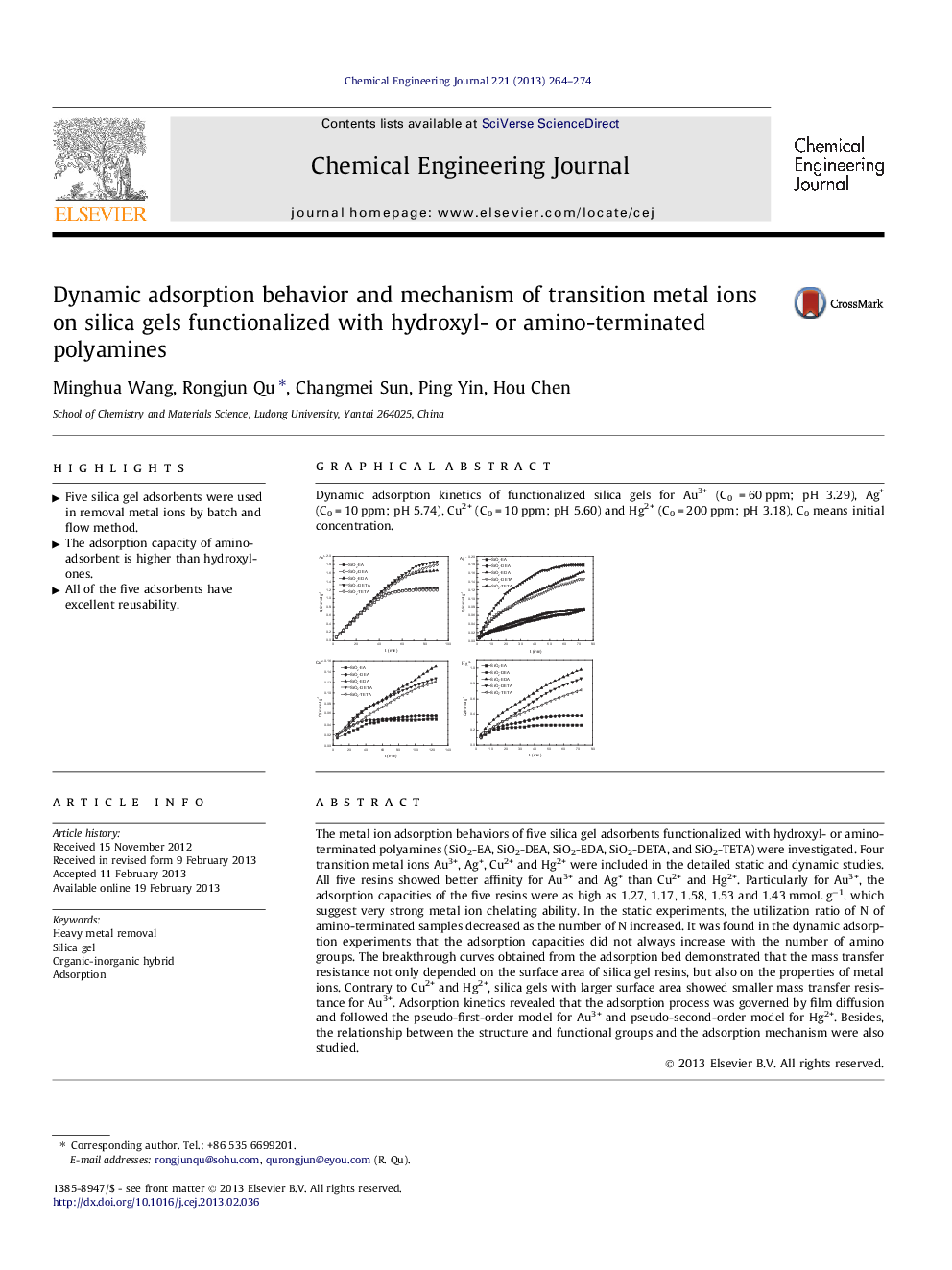
The metal ion adsorption behaviors of five silica gel adsorbents functionalized with hydroxyl- or amino-terminated polyamines (SiO2-EA, SiO2-DEA, SiO2-EDA, SiO2-DETA, and SiO2-TETA) were investigated. Four transition metal ions Au3+, Ag+, Cu2+ and Hg2+ were included in the detailed static and dynamic studies. All five resins showed better affinity for Au3+ and Ag+ than Cu2+ and Hg2+. Particularly for Au3+, the adsorption capacities of the five resins were as high as 1.27, 1.17, 1.58, 1.53 and 1.43 mmoL g−1, which suggest very strong metal ion chelating ability. In the static experiments, the utilization ratio of N of amino-terminated samples decreased as the number of N increased. It was found in the dynamic adsorption experiments that the adsorption capacities did not always increase with the number of amino groups. The breakthrough curves obtained from the adsorption bed demonstrated that the mass transfer resistance not only depended on the surface area of silica gel resins, but also on the properties of metal ions. Contrary to Cu2+ and Hg2+, silica gels with larger surface area showed smaller mass transfer resistance for Au3+. Adsorption kinetics revealed that the adsorption process was governed by film diffusion and followed the pseudo-first-order model for Au3+ and pseudo-second-order model for Hg2+. Besides, the relationship between the structure and functional groups and the adsorption mechanism were also studied.
Dynamic adsorption kinetics of functionalized silica gels for Au3+ (C0 = 60 ppm; pH 3.29), Ag+ (C0 = 10 ppm; pH 5.74), Cu2+ (C0 = 10 ppm; pH 5.60) and Hg2+ (C0 = 200 ppm; pH 3.18), C0 means initial concentration.Figure optionsDownload as PowerPoint slideHighlights
► Five silica gel adsorbents were used in removal metal ions by batch and flow method.
► The adsorption capacity of amino-adsorbent is higher than hydroxyl-ones.
► All of the five adsorbents have excellent reusability.
Journal: Chemical Engineering Journal - Volume 221, 1 April 2013, Pages 264–274