| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 149167 | 456428 | 2012 | 8 صفحه PDF | دانلود رایگان |
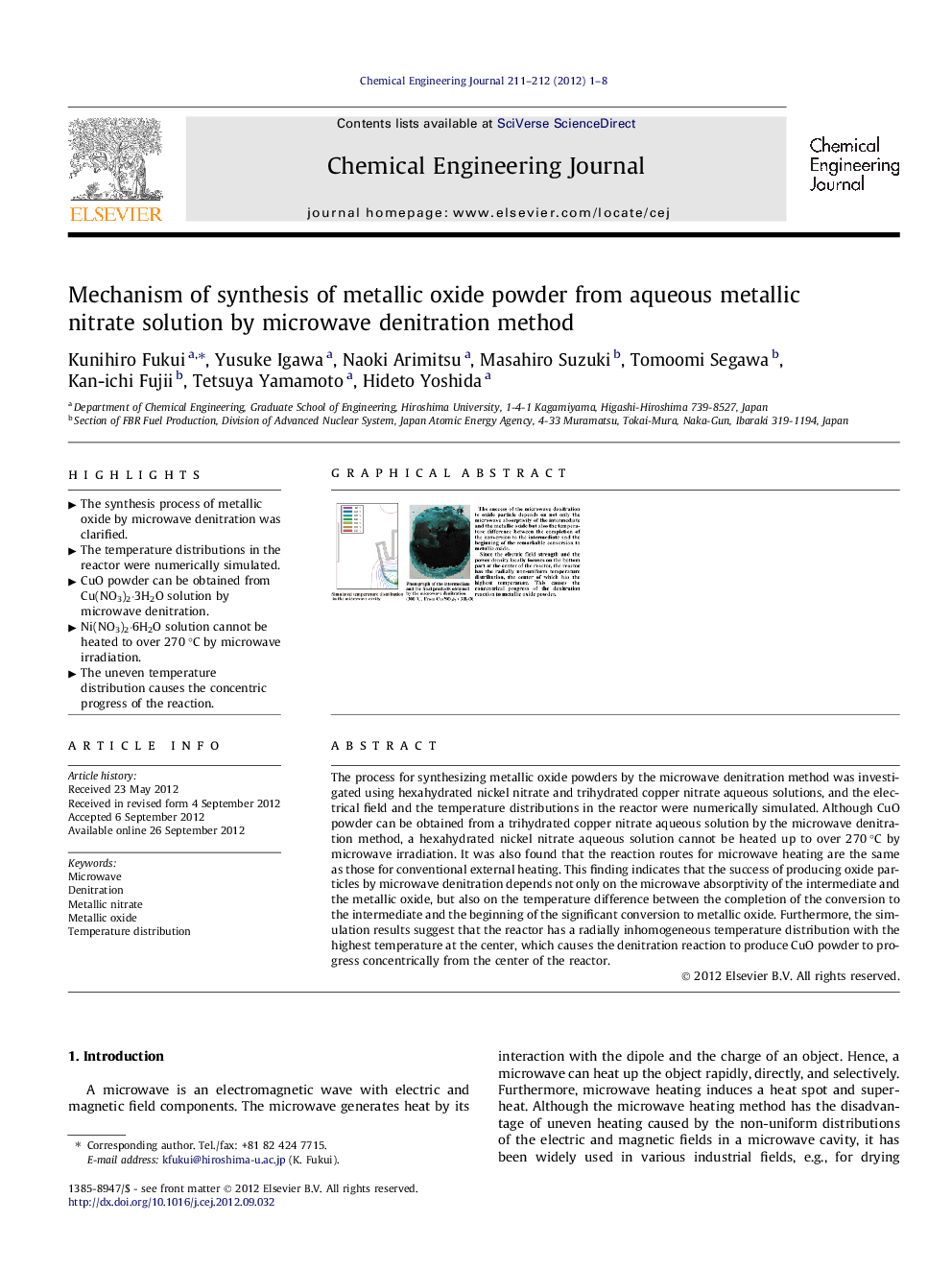
The process for synthesizing metallic oxide powders by the microwave denitration method was investigated using hexahydrated nickel nitrate and trihydrated copper nitrate aqueous solutions, and the electrical field and the temperature distributions in the reactor were numerically simulated. Although CuO powder can be obtained from a trihydrated copper nitrate aqueous solution by the microwave denitration method, a hexahydrated nickel nitrate aqueous solution cannot be heated up to over 270 °C by microwave irradiation. It was also found that the reaction routes for microwave heating are the same as those for conventional external heating. This finding indicates that the success of producing oxide particles by microwave denitration depends not only on the microwave absorptivity of the intermediate and the metallic oxide, but also on the temperature difference between the completion of the conversion to the intermediate and the beginning of the significant conversion to metallic oxide. Furthermore, the simulation results suggest that the reactor has a radially inhomogeneous temperature distribution with the highest temperature at the center, which causes the denitration reaction to produce CuO powder to progress concentrically from the center of the reactor.
Figure optionsDownload as PowerPoint slideHighlights
► The synthesis process of metallic oxide by microwave denitration was clarified.
► The temperature distributions in the reactor were numerically simulated.
► CuO powder can be obtained from Cu(NO3)2⋅3H2O solution by microwave denitration.
► Ni(NO3)2⋅6H2O solution cannot be heated to over 270 °C by microwave irradiation.
► The uneven temperature distribution causes the concentric progress of the reaction.
Journal: Chemical Engineering Journal - Volumes 211–212, 15 November 2012, Pages 1–8