| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 168911 | 1423446 | 2009 | 10 صفحه PDF | دانلود رایگان |
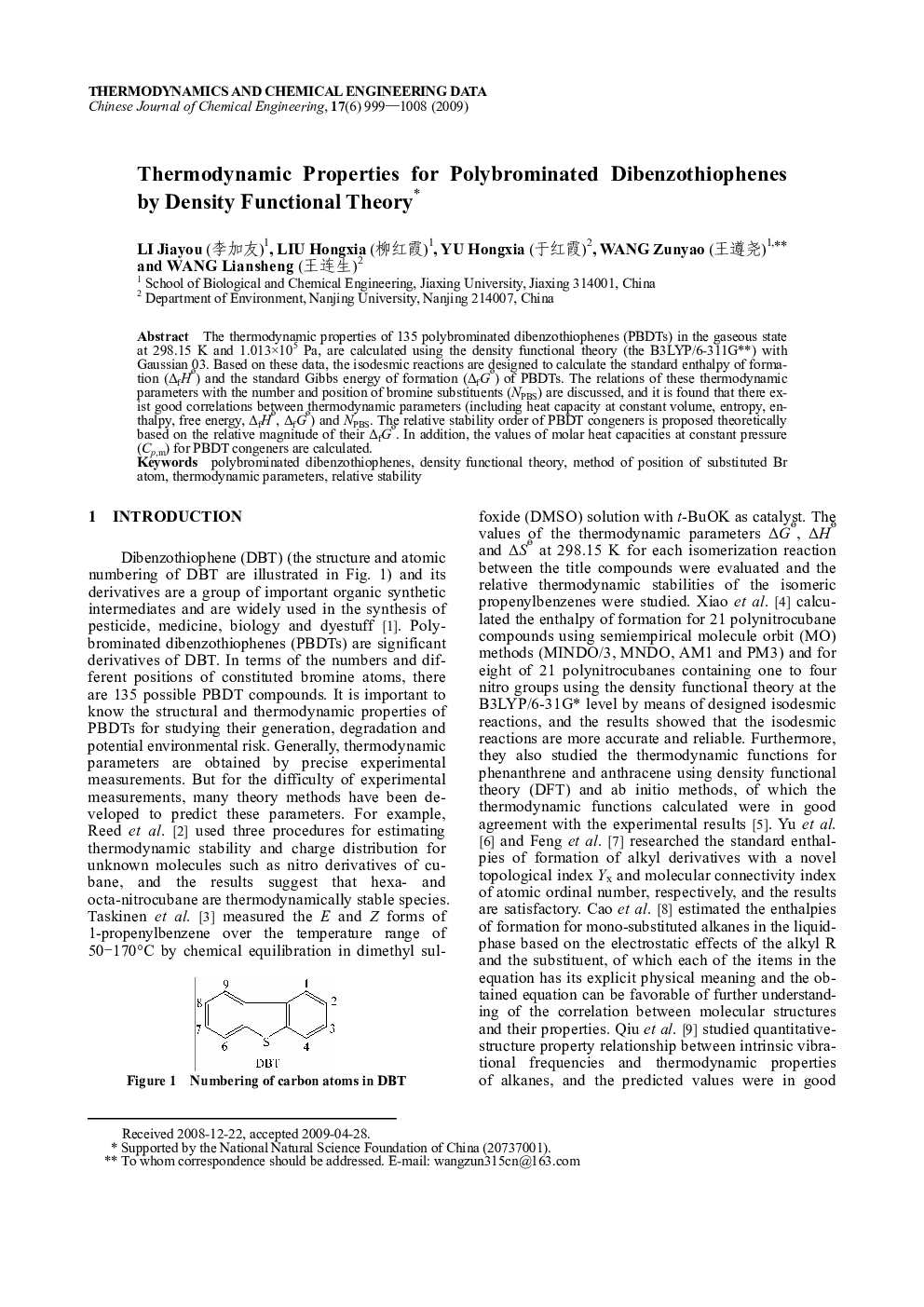
The thermodynamic properties of 135 polybrominated dibenzothiophenes (PBDTs) in the gaseous state at 298.15 K and 1.013×105 Pa, are calculated using the density functional theory (the B3LYP/6–311G**) with Gaussian 03. Based on these data, the isodesmic reactions are designed to calculate the standard enthalpy of formation (ΔfHθ) and the standard Gibbs energy of formation (ΔfGθ) of PBDTs. The relations of these thermodynamic parameters with the number and position of bromine substituents (NPBS) are discussed, and it is found that there exist good correlations between thermodynamic parameters (including heat capacity at constant volume, entropy, enthalpy, free energy, ΔfHθ, ΔfGθ) and NPBS. The relative stability order of PBDT congeners is proposed theoretically based on the relative magnitude of their ΔfGθ. In addition, the values of molar heat capacities at constant pressure (Cp,m) for PBDT congeners are calculated.
Journal: Chinese Journal of Chemical Engineering - Volume 17, Issue 6, December 2009, Pages 999-1008