| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5218054 | 1383314 | 2013 | 10 صفحه PDF | دانلود رایگان |
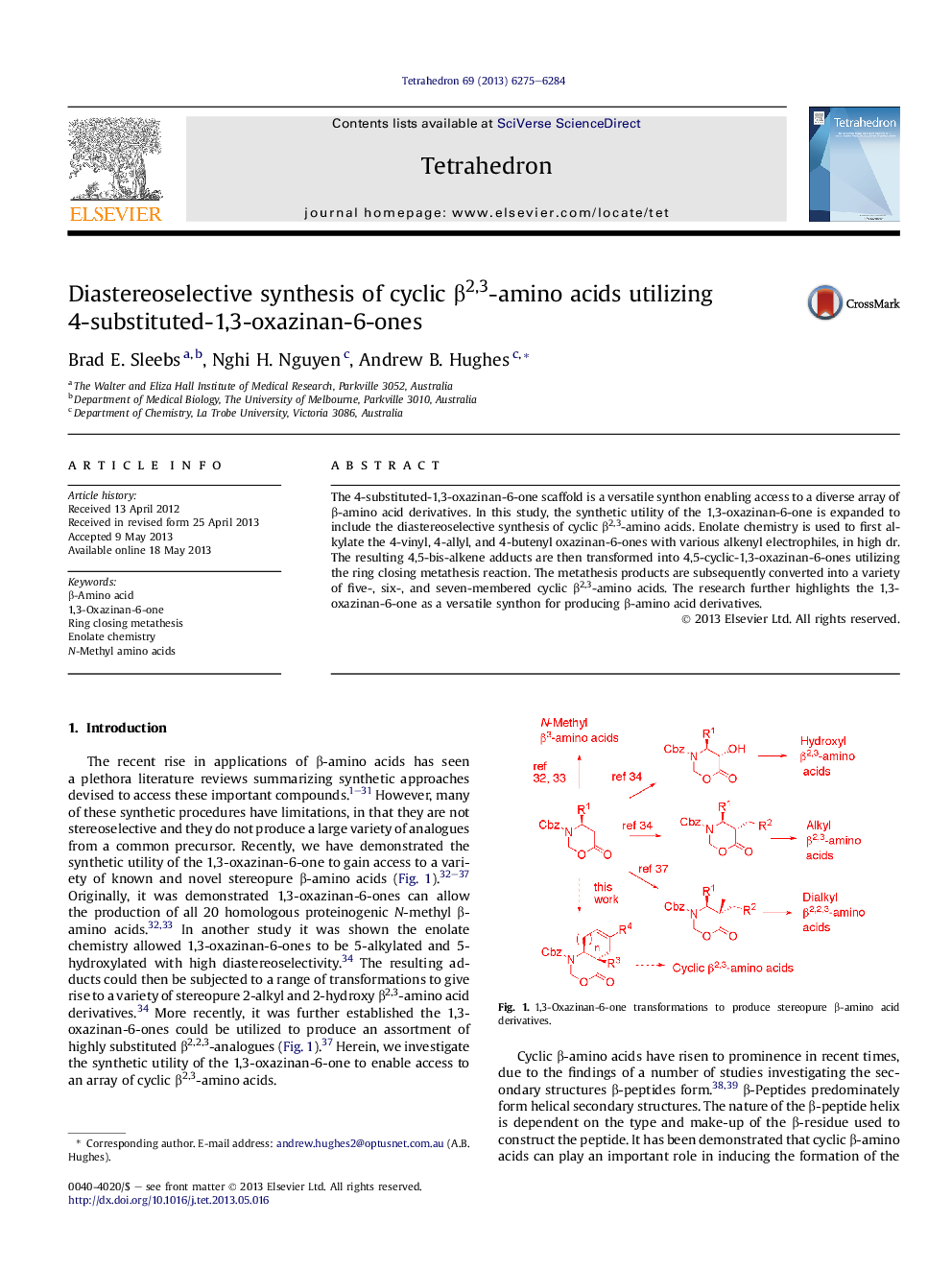
The 4-substituted-1,3-oxazinan-6-one scaffold is a versatile synthon enabling access to a diverse array of β-amino acid derivatives. In this study, the synthetic utility of the 1,3-oxazinan-6-one is expanded to include the diastereoselective synthesis of cyclic β2,3-amino acids. Enolate chemistry is used to first alkylate the 4-vinyl, 4-allyl, and 4-butenyl oxazinan-6-ones with various alkenyl electrophiles, in high dr. The resulting 4,5-bis-alkene adducts are then transformed into 4,5-cyclic-1,3-oxazinan-6-ones utilizing the ring closing metathesis reaction. The metathesis products are subsequently converted into a variety of five-, six-, and seven-membered cyclic β2,3-amino acids. The research further highlights the 1,3-oxazinan-6-one as a versatile synthon for producing β-amino acid derivatives.
Journal: Tetrahedron - Volume 69, Issue 30, 29 July 2013, Pages 6275-6284