| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5222473 | 1383456 | 2009 | 11 صفحه PDF | دانلود رایگان |
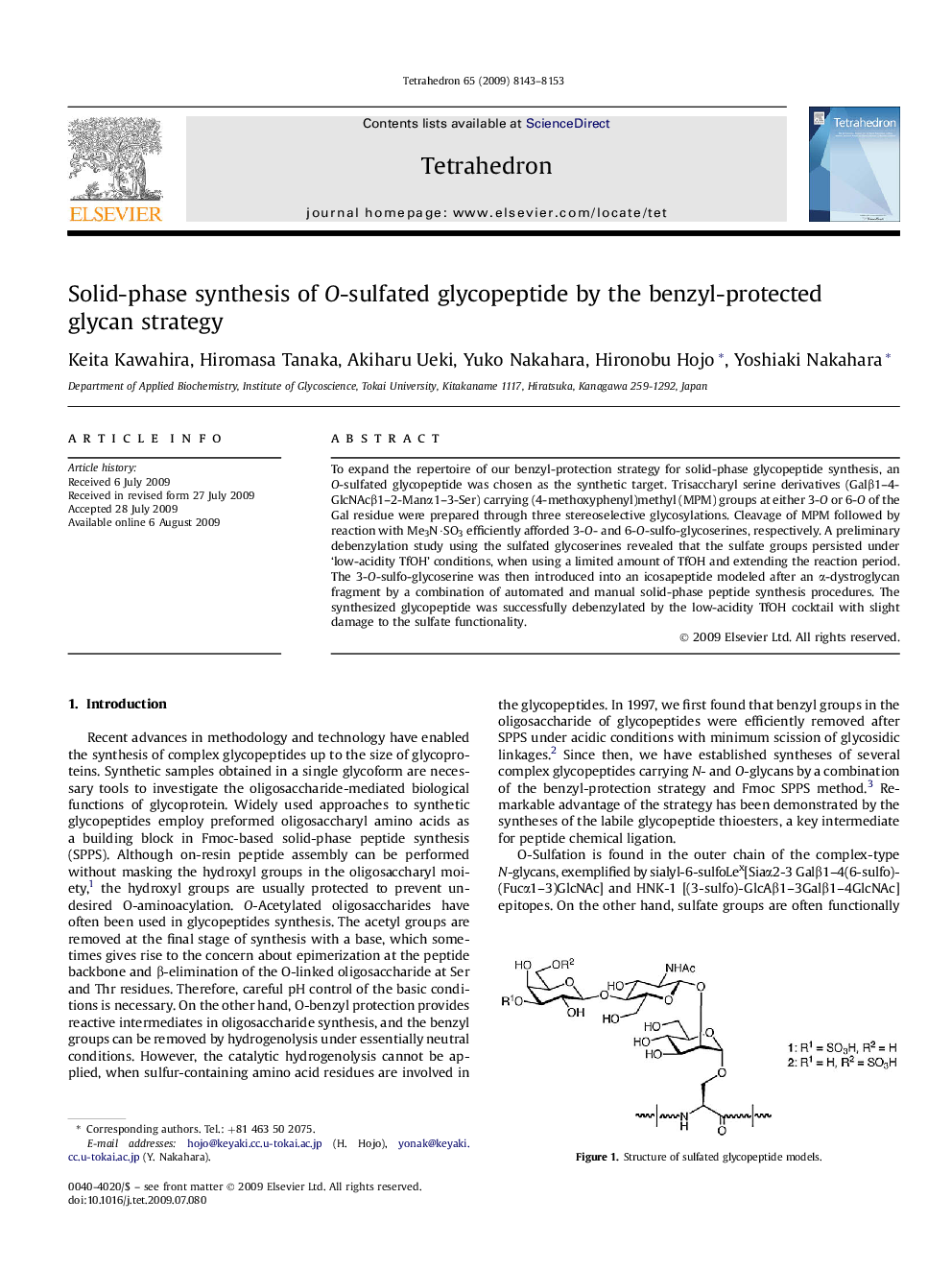
To expand the repertoire of our benzyl-protection strategy for solid-phase glycopeptide synthesis, an O-sulfated glycopeptide was chosen as the synthetic target. Trisaccharyl serine derivatives (Galβ1-4-GlcNAcβ1-2-Manα1-3-Ser) carrying (4-methoxyphenyl)methyl (MPM) groups at either 3-O or 6-O of the Gal residue were prepared through three stereoselective glycosylations. Cleavage of MPM followed by reaction with Me3N·SO3 efficiently afforded 3-O- and 6-O-sulfo-glycoserines, respectively. A preliminary debenzylation study using the sulfated glycoserines revealed that the sulfate groups persisted under 'low-acidity TfOH' conditions, when using a limited amount of TfOH and extending the reaction period. The 3-O-sulfo-glycoserine was then introduced into an icosapeptide modeled after an α-dystroglycan fragment by a combination of automated and manual solid-phase peptide synthesis procedures. The synthesized glycopeptide was successfully debenzylated by the low-acidity TfOH cocktail with slight damage to the sulfate functionality.
Journal: Tetrahedron - Volume 65, Issue 39, 26 September 2009, Pages 8143-8153