| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 54925 | 47032 | 2013 | 10 صفحه PDF | دانلود رایگان |
The effect of Pd addition over an industrial catalyst Pt/Ba/CeO2/Al2O3 in the lean-NOx trap process was investigated. FTIR operando was carried out for an in depth understanding of the chemical mechanisms taking place onto the catalyst in working conditions close to the real ones. The presence of Pd provokes a synergetic effect, probably related to a Pt–Pd alloy that improves the NO oxidation and therefore the NOx trapping capacity. Besides, Pd also favours the hydrocarbon oxidation and thus increases the efficiency of the process. On the basis of the present investigations and previous works from the literature, a reaction mechanism is proposed.
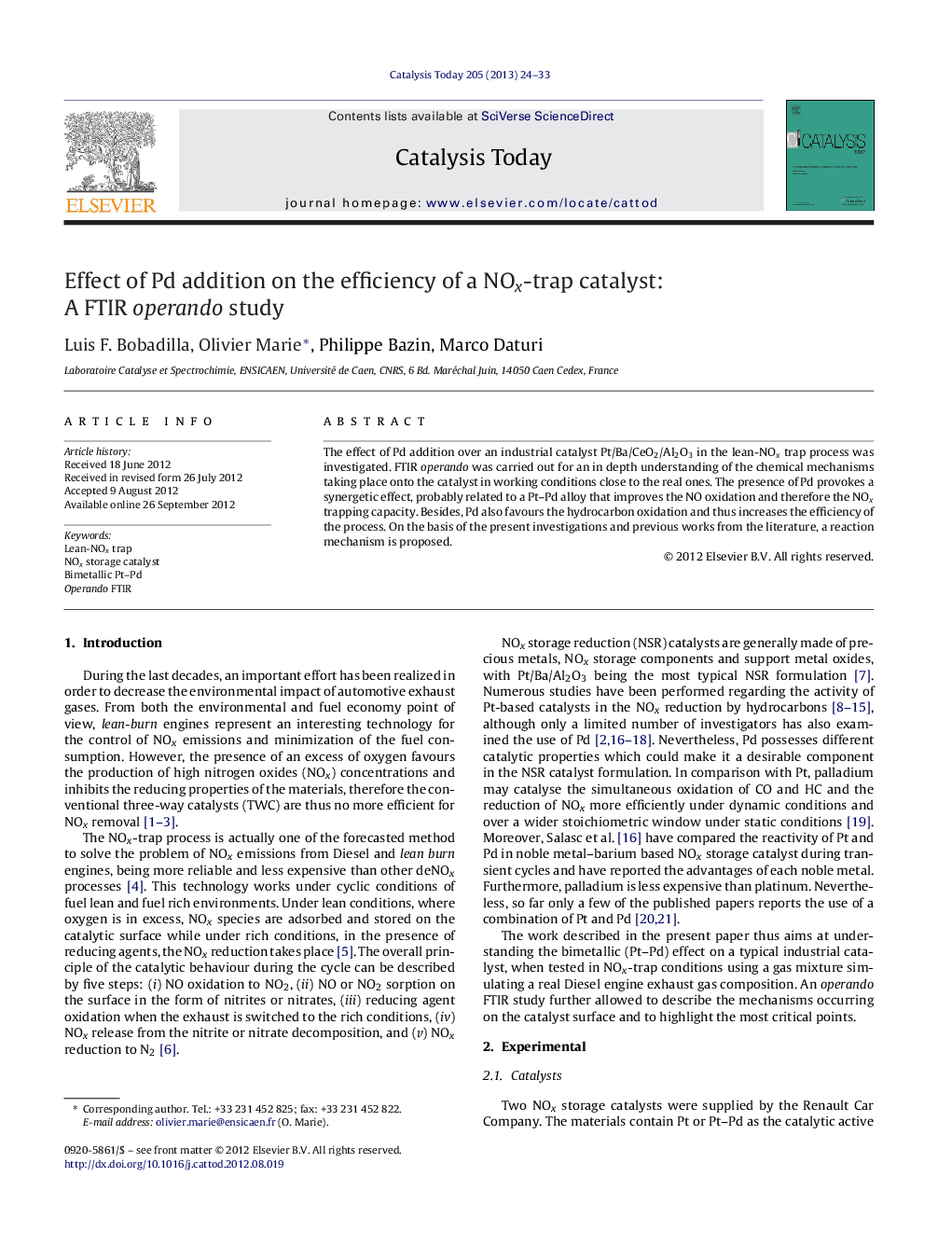
A model for the positive Pd effect. Pd favours the HC oxidation and prevents the Pt poisoning by CO at intermediate temperatures.Figure optionsDownload high-quality image (136 K)Download as PowerPoint slideHighlights
► Pd addition to a Pt-catalyst inhibits CO adsorption below 250 °C probably through the formation of a Pt–Pd alloy.
► A bimetallic catalyst is thus much more efficient in the NO to NO2 oxidation and in NOx storage capacity.
► Stabilization under rich flow (CO, H2 and C10H22) leads to acrylates, formates and acetates deposit detected by IR operando.
► Under lean flow, pre-adsorbed formates and acetates (more abundant over Pt–Pd catalyst) are initially involved in NOx SCR.
► Under lean/rich cycles, the progressive NOx removal efficiency lost at 280 °C is due to the accumulation of stable nitrates.
Journal: Catalysis Today - Volume 205, 30 April 2013, Pages 24–33