| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593979 | 1453959 | 2012 | 11 صفحه PDF | دانلود رایگان |
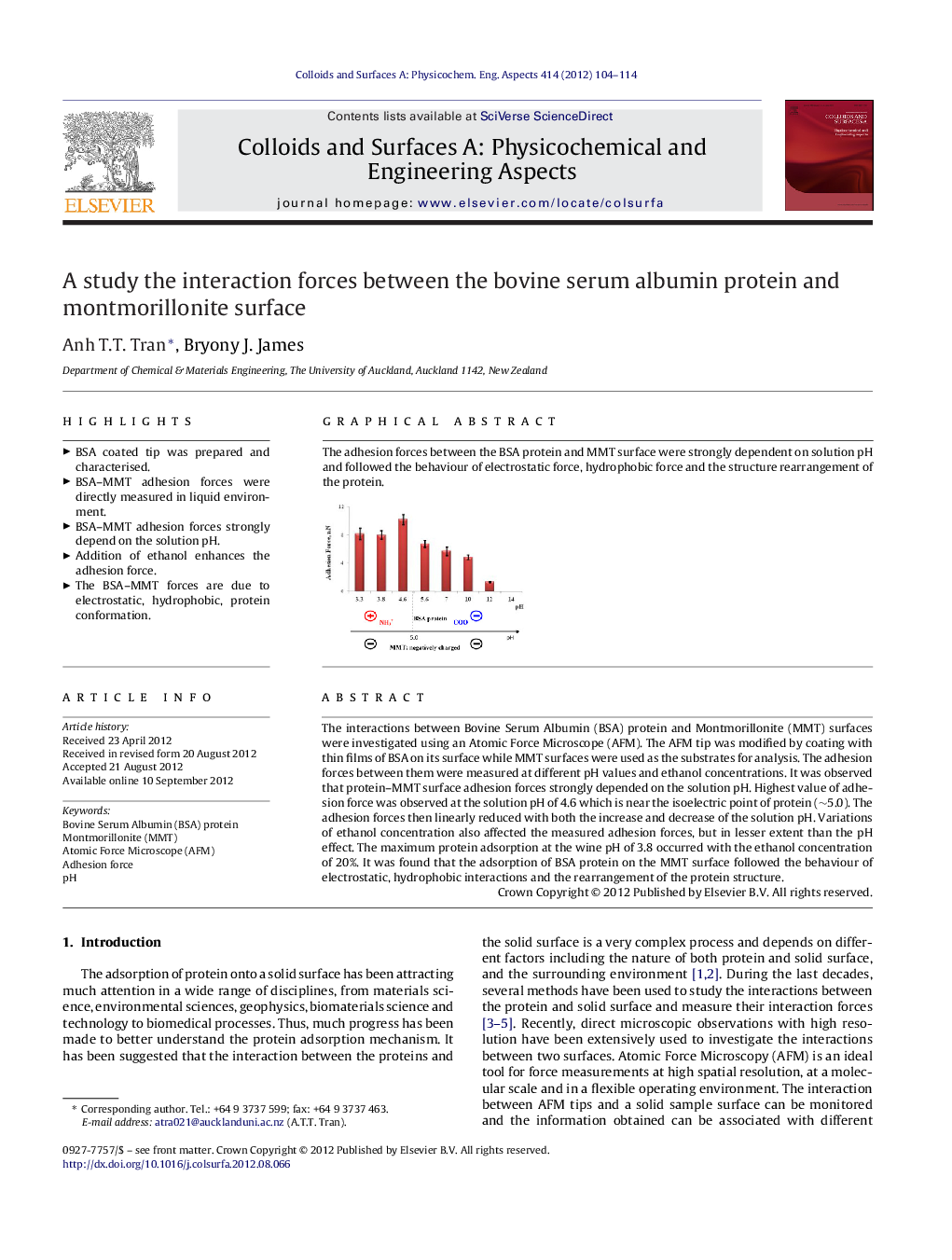
The interactions between Bovine Serum Albumin (BSA) protein and Montmorillonite (MMT) surfaces were investigated using an Atomic Force Microscope (AFM). The AFM tip was modified by coating with thin films of BSA on its surface while MMT surfaces were used as the substrates for analysis. The adhesion forces between them were measured at different pH values and ethanol concentrations. It was observed that protein–MMT surface adhesion forces strongly depended on the solution pH. Highest value of adhesion force was observed at the solution pH of 4.6 which is near the isoelectric point of protein (∼5.0). The adhesion forces then linearly reduced with both the increase and decrease of the solution pH. Variations of ethanol concentration also affected the measured adhesion forces, but in lesser extent than the pH effect. The maximum protein adsorption at the wine pH of 3.8 occurred with the ethanol concentration of 20%. It was found that the adsorption of BSA protein on the MMT surface followed the behaviour of electrostatic, hydrophobic interactions and the rearrangement of the protein structure.
The adhesion forces between the BSA protein and MMT surface were strongly dependent on solution pH and followed the behaviour of electrostatic force, hydrophobic force and the structure rearrangement of the protein.Figure optionsDownload as PowerPoint slideHighlights
► BSA coated tip was prepared and characterised.
► BSA–MMT adhesion forces were directly measured in liquid environment.
► BSA–MMT adhesion forces strongly depend on the solution pH.
► Addition of ethanol enhances the adhesion force.
► The BSA–MMT forces are due to electrostatic, hydrophobic, protein conformation.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 414, 20 November 2012, Pages 104–114