| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593989 | 1453959 | 2012 | 10 صفحه PDF | دانلود رایگان |
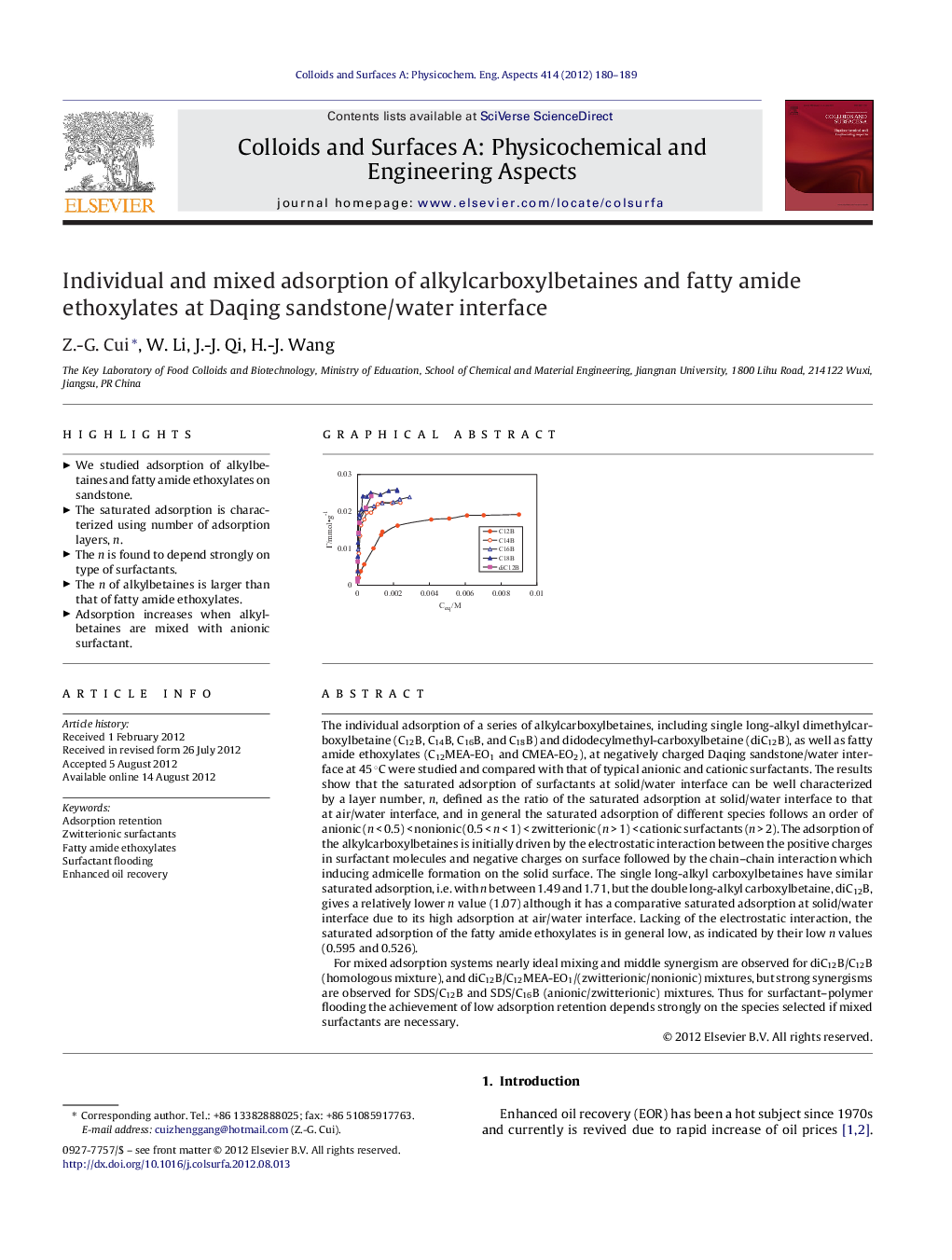
The individual adsorption of a series of alkylcarboxylbetaines, including single long-alkyl dimethylcarboxylbetaine (C12B, C14B, C16B, and C18B) and didodecylmethyl-carboxylbetaine (diC12B), as well as fatty amide ethoxylates (C12MEA-EO1 and CMEA-EO2), at negatively charged Daqing sandstone/water interface at 45 °C were studied and compared with that of typical anionic and cationic surfactants. The results show that the saturated adsorption of surfactants at solid/water interface can be well characterized by a layer number, n, defined as the ratio of the saturated adsorption at solid/water interface to that at air/water interface, and in general the saturated adsorption of different species follows an order of anionic (n < 0.5) < nonionic (0.5 < n < 1) < zwitterionic (n > 1) < cationic surfactants (n > 2). The adsorption of the alkylcarboxylbetaines is initially driven by the electrostatic interaction between the positive charges in surfactant molecules and negative charges on surface followed by the chain–chain interaction which inducing admicelle formation on the solid surface. The single long-alkyl carboxylbetaines have similar saturated adsorption, i.e. with n between 1.49 and 1.71, but the double long-alkyl carboxylbetaine, diC12B, gives a relatively lower n value (1.07) although it has a comparative saturated adsorption at solid/water interface due to its high adsorption at air/water interface. Lacking of the electrostatic interaction, the saturated adsorption of the fatty amide ethoxylates is in general low, as indicated by their low n values (0.595 and 0.526).For mixed adsorption systems nearly ideal mixing and middle synergism are observed for diC12B/C12B (homologous mixture), and diC12B/C12MEA-EO1/(zwitterionic/nonionic) mixtures, but strong synergisms are observed for SDS/C12B and SDS/C16B (anionic/zwitterionic) mixtures. Thus for surfactant–polymer flooding the achievement of low adsorption retention depends strongly on the species selected if mixed surfactants are necessary.
Figure optionsDownload as PowerPoint slideHighlights
► We studied adsorption of alkylbetaines and fatty amide ethoxylates on sandstone.
► The saturated adsorption is characterized using number of adsorption layers, n.
► The n is found to depend strongly on type of surfactants.
► The n of alkylbetaines is larger than that of fatty amide ethoxylates.
► Adsorption increases when alkylbetaines are mixed with anionic surfactant.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 414, 20 November 2012, Pages 180–189