| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 594021 | 1453959 | 2012 | 11 صفحه PDF | دانلود رایگان |
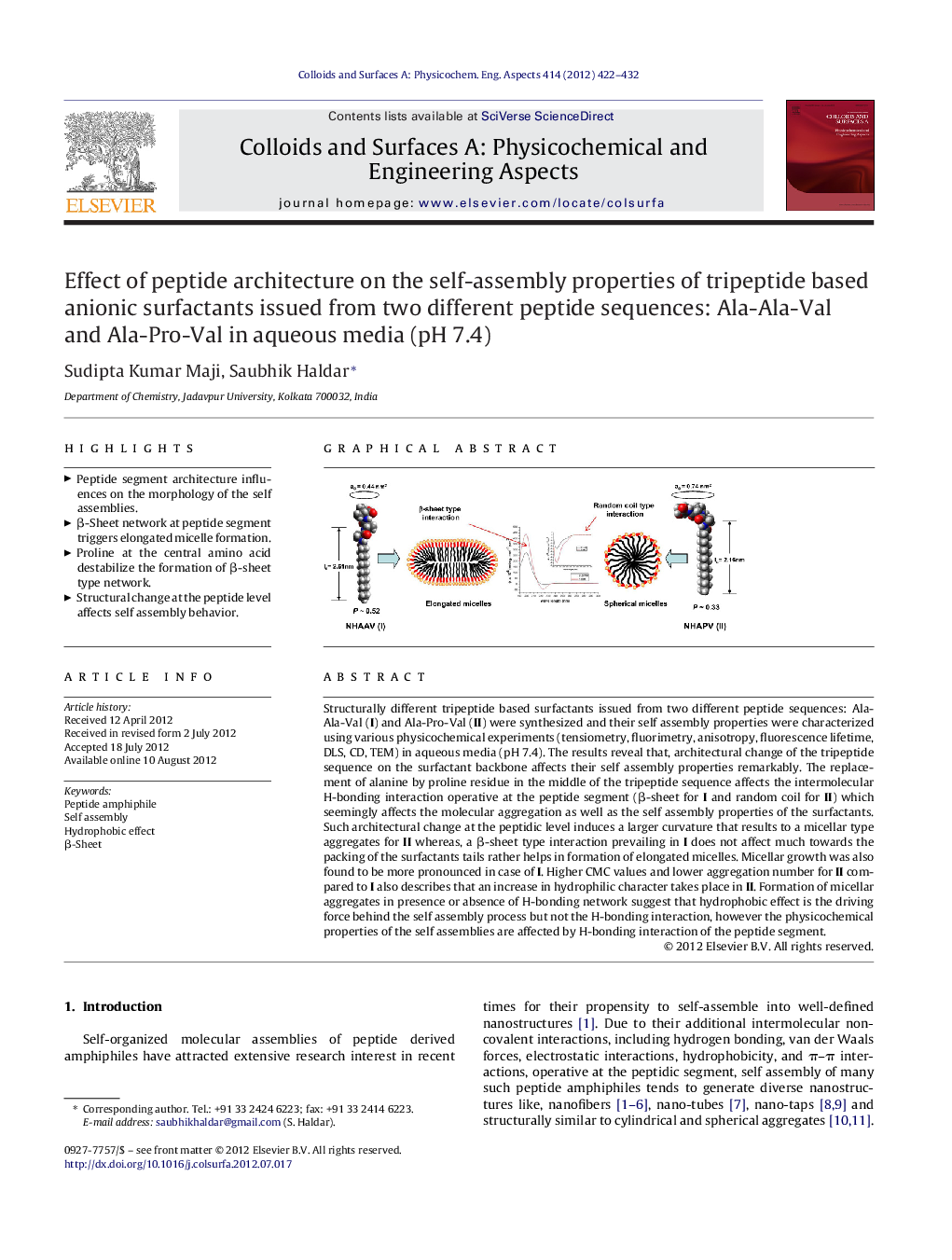
Structurally different tripeptide based surfactants issued from two different peptide sequences: Ala-Ala-Val (I) and Ala-Pro-Val (II) were synthesized and their self assembly properties were characterized using various physicochemical experiments (tensiometry, fluorimetry, anisotropy, fluorescence lifetime, DLS, CD, TEM) in aqueous media (pH 7.4). The results reveal that, architectural change of the tripeptide sequence on the surfactant backbone affects their self assembly properties remarkably. The replacement of alanine by proline residue in the middle of the tripeptide sequence affects the intermolecular H-bonding interaction operative at the peptide segment (β-sheet for I and random coil for II) which seemingly affects the molecular aggregation as well as the self assembly properties of the surfactants. Such architectural change at the peptidic level induces a larger curvature that results to a micellar type aggregates for II whereas, a β-sheet type interaction prevailing in I does not affect much towards the packing of the surfactants tails rather helps in formation of elongated micelles. Micellar growth was also found to be more pronounced in case of I. Higher CMC values and lower aggregation number for II compared to I also describes that an increase in hydrophilic character takes place in II. Formation of micellar aggregates in presence or absence of H-bonding network suggest that hydrophobic effect is the driving force behind the self assembly process but not the H-bonding interaction, however the physicochemical properties of the self assemblies are affected by H-bonding interaction of the peptide segment.
Figure optionsDownload as PowerPoint slideHighlights
► Peptide segment architecture influences on the morphology of the self assemblies.
► β-Sheet network at peptide segment triggers elongated micelle formation.
► Proline at the central amino acid destabilize the formation of β-sheet type network.
► Structural change at the peptide level affects self assembly behavior.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 414, 20 November 2012, Pages 422–432