| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 612115 | 880691 | 2007 | 6 صفحه PDF | دانلود رایگان |
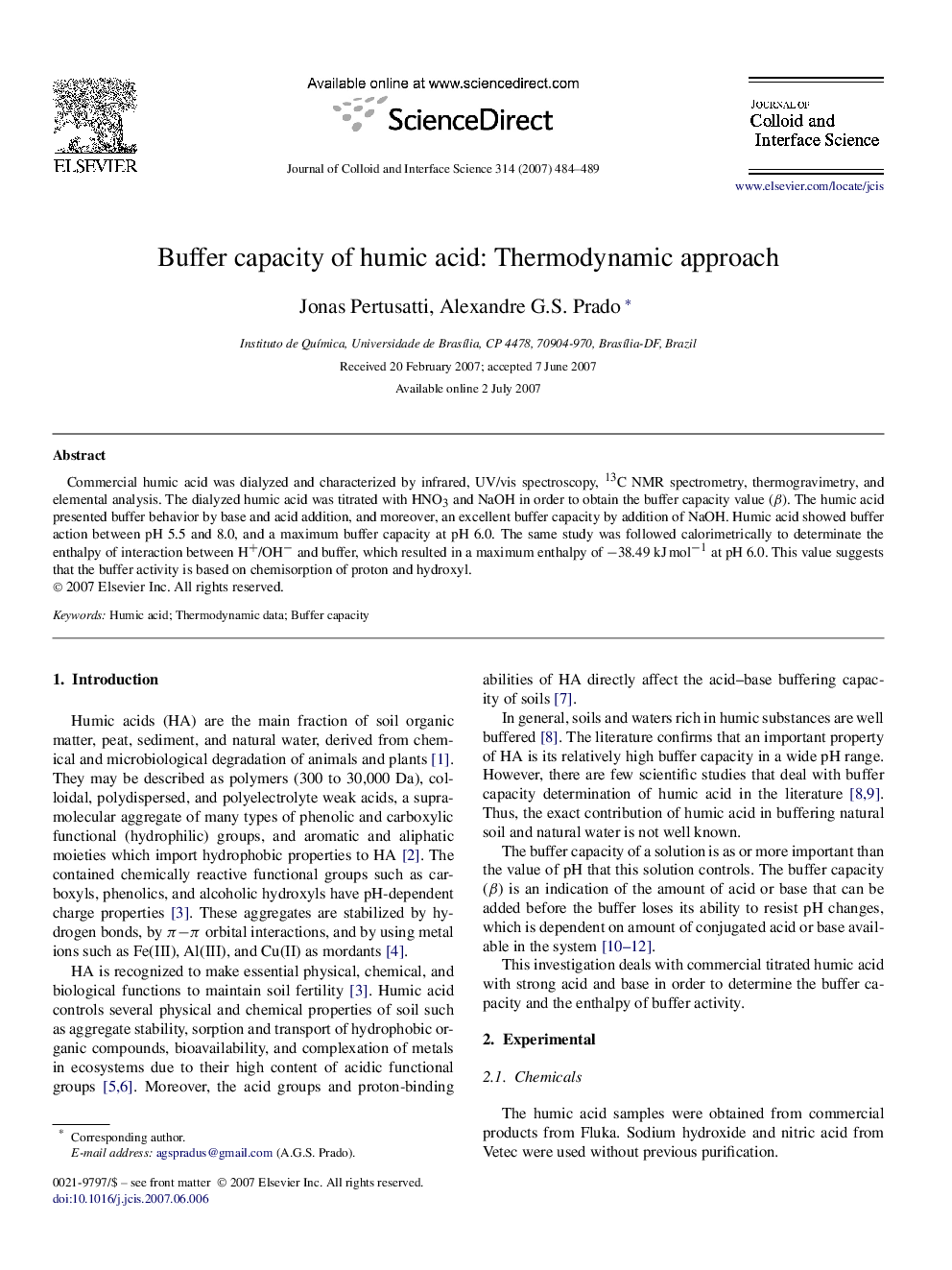
Commercial humic acid was dialyzed and characterized by infrared, UV/vis spectroscopy, 13C NMR spectrometry, thermogravimetry, and elemental analysis. The dialyzed humic acid was titrated with HNO3 and NaOH in order to obtain the buffer capacity value (β). The humic acid presented buffer behavior by base and acid addition, and moreover, an excellent buffer capacity by addition of NaOH. Humic acid showed buffer action between pH 5.5 and 8.0, and a maximum buffer capacity at pH 6.0. The same study was followed calorimetrically to determinate the enthalpy of interaction between H+/OH− and buffer, which resulted in a maximum enthalpy of −38.49 kJ mol−1 at pH 6.0. This value suggests that the buffer activity is based on chemisorption of proton and hydroxyl.
Buffer capacity variation (○) and the heat (■) versus pH range.Figure optionsDownload as PowerPoint slide
Journal: Journal of Colloid and Interface Science - Volume 314, Issue 2, 15 October 2007, Pages 484–489