| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 66500 | 48435 | 2011 | 7 صفحه PDF | دانلود رایگان |
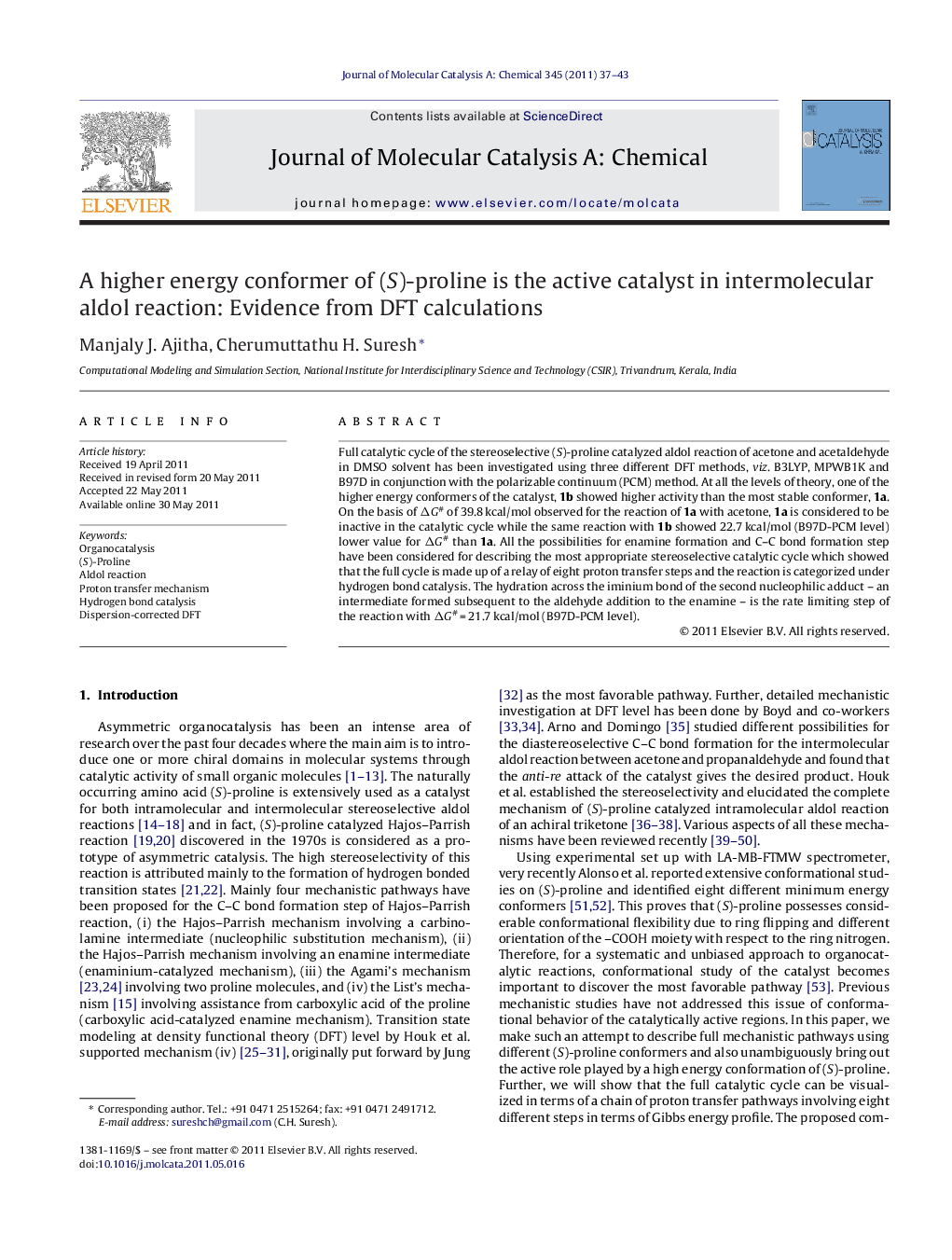
Full catalytic cycle of the stereoselective (S)-proline catalyzed aldol reaction of acetone and acetaldehyde in DMSO solvent has been investigated using three different DFT methods, viz. B3LYP, MPWB1K and B97D in conjunction with the polarizable continuum (PCM) method. At all the levels of theory, one of the higher energy conformers of the catalyst, 1b showed higher activity than the most stable conformer, 1a. On the basis of ΔG# of 39.8 kcal/mol observed for the reaction of 1a with acetone, 1a is considered to be inactive in the catalytic cycle while the same reaction with 1b showed 22.7 kcal/mol (B97D-PCM level) lower value for ΔG# than 1a. All the possibilities for enamine formation and C–C bond formation step have been considered for describing the most appropriate stereoselective catalytic cycle which showed that the full cycle is made up of a relay of eight proton transfer steps and the reaction is categorized under hydrogen bond catalysis. The hydration across the iminium bond of the second nucleophilic adduct – an intermediate formed subsequent to the aldehyde addition to the enamine – is the rate limiting step of the reaction with ΔG# = 21.7 kcal/mol (B97D-PCM level).
(S)-Proline mediated aldol reaction passes through eight proton transfer transition states. A higher energy conformer of (S)-proline (1b) is the active form in mediating the reaction and hydration across iminium bond after a second nucleophilic attack is found to be the rate determining stage of the reaction.Figure optionsDownload high-quality image (207 K)Download as PowerPoint slideHighlights
► Theoretical revisiting of (S)-proline catalyzed intermolecular aldol reaction.
► Higher energy conformer of (S)-proline is found to be the active catalyst.
► The mechanism passes through eight proton transfer transition states.
► Hydrogen bond catalysis.
Journal: Journal of Molecular Catalysis A: Chemical - Volume 345, Issues 1–2, 5 July 2011, Pages 37–43