| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1228896 | 1495225 | 2015 | 11 صفحه PDF | دانلود رایگان |
• A detailed vibrational assignments of experimental and theoretical wave numbers of HPCI were reported.
• Optimized geometrical parameters of HPCI on B3LYP/6-311+G(d,p) level along with experimental were performed.
• Calculated electronic absorption spectral data were reported.
• NBO, HOMO and LUMO analysis were also performed by DFT.
• Kinetic and thermodynamic stabilities of the molecules were determined.
The FT-IR, FT-Raman, 1H, 13C NMR and UV–Visible spectral measurements of N′-hydroxy-pyrimidine-2-carboximidamide (HPCI) and complete analysis of the observed spectra have been proposed. DFT calculation has been performed and the structural parameters of the compound was determined from the optimized geometry with 6-311+G(d,p) basis set and giving energies, harmonic vibrational frequencies and force constants. The results of the optimized molecular structure are presented and compared with the experimental. The geometric parameters, harmonic vibrational frequencies and chemical shifts were compared with the experimental data of the molecule. The title compound, C5H6N4O, is approximately planar, with an angle of 11.04 (15)°. The crystal structure is also stabilized by intermolecular N–H⋯O, N–H⋯N, O–H⋯N, C–H⋯O hydrogen bond and offset π–π stacking interactions. The influences of hydroxy and carboximidamide groups on the skeletal modes and proton chemical shifts have been investigated. Moreover, we have not only simulated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) but also determined the transition state and band gap. The kinetic, thermodynamic stability and chemical hardness of the molecule have been determined. Complete NBO analysis was also carried out to find out the intermolecular electronic interactions and their stabilization energy. The thermodynamic properties like entropies and their correlations with temperatures were also obtained from the harmonic frequencies of the optimized structure.
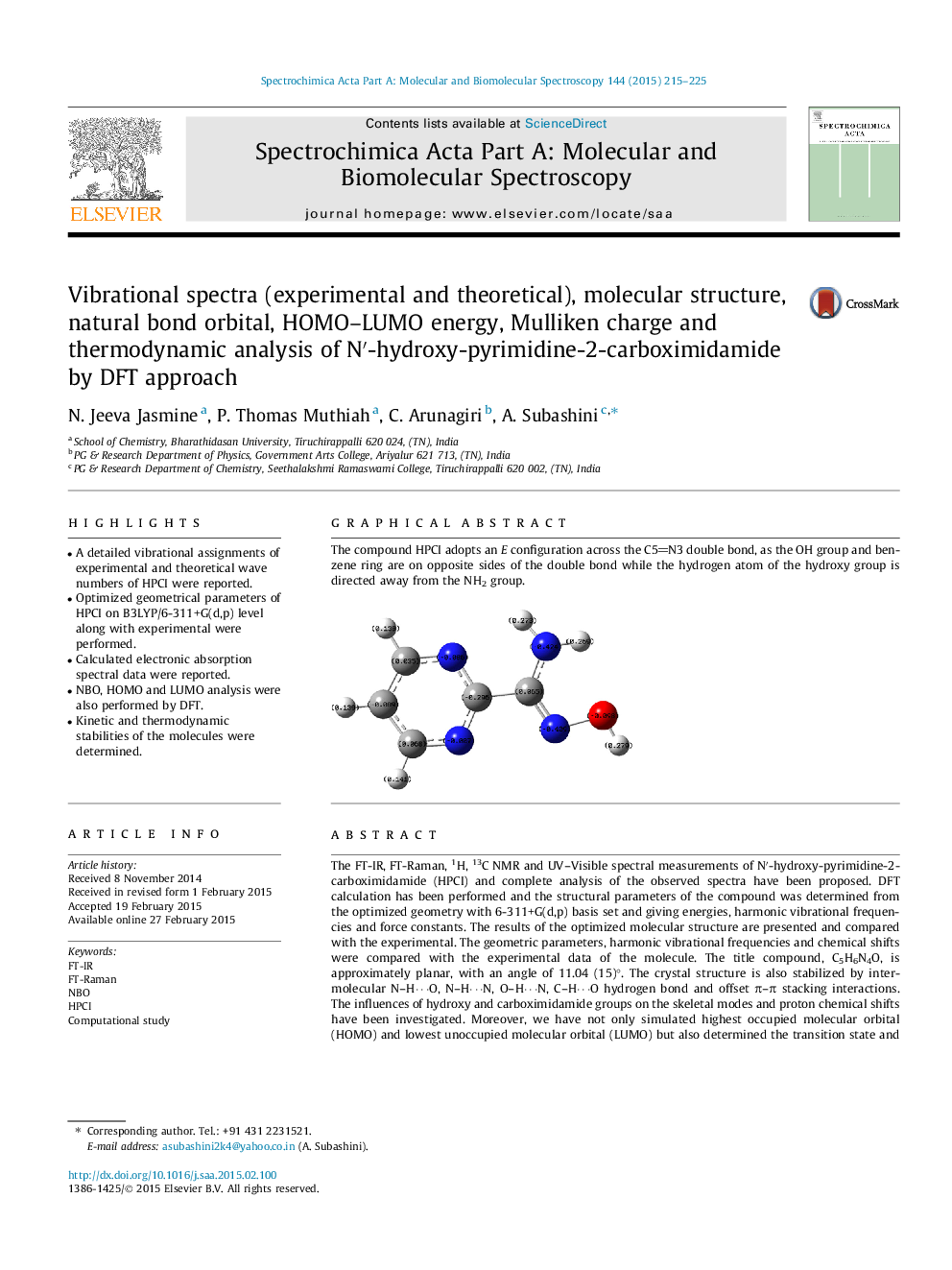
The compound HPCI adopts an E configuration across the C5N3 double bond, as the OH group and benzene ring are on opposite sides of the double bond while the hydrogen atom of the hydroxy group is directed away from the NH2 group.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 144, 5 June 2015, Pages 215–225