| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230706 | 1495261 | 2013 | 13 صفحه PDF | دانلود رایگان |
The experimental and theoretical vibrational spectra of S-S-2 methylamino-1-phenyl propan-1-ol (SSMPL). Fourier transform infrared (FTIR) and FT Raman spectra of SSMPL in the solid phase were recorded and analyzed. The molecular geometry, vibrational frequencies, infrared intensities, Raman activities and atomic charges were calculated using density functional theory calculation (B3LYP) with standard 6-31G(d,p) and high level 6-311++G(d,p) basis sets. Complete vibrational assignment and analysis of the fundamental modes of the compound were carried out using the observed FTIR and FT Raman data. The thermodynamic functions of the title compound were also performed by B3LYP with two basis sets 6-31G(d,p) and 6-311++G(d,p). Stability of the molecule arising from hyper conjugative interactions, charge delocalization has been analyzed using natural bond orbital (NBO) analysis. The calculated HOMO and LUMO energies show that charge transfer occurs within the molecule. Using the method B3LYP, the dipole moment (μ), polarizability (α) and the hyperpolarizability (β) values of the investigated molecule has been computed. Total energy distribution (TED) was used for the assignment of Unambiguous vibrational fundamental modes. Finally, Simulated FTIR and FT Raman spectra of SSMPL showed good agreement with the observed spectra.
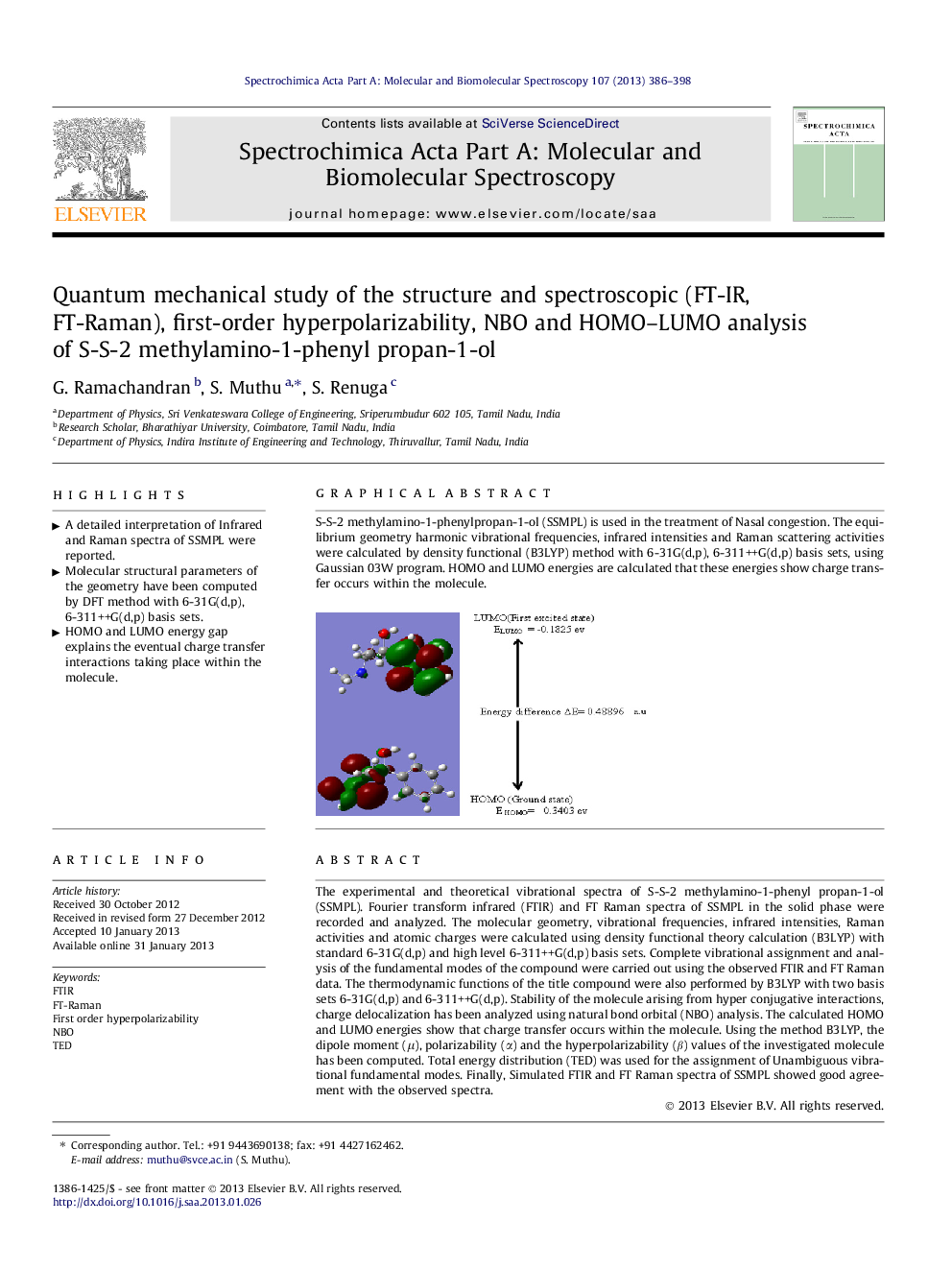
S-S-2 methylamino-1-phenylpropan-1-ol (SSMPL) is used in the treatment of Nasal congestion. The equilibrium geometry harmonic vibrational frequencies, infrared intensities and Raman scattering activities were calculated by density functional (B3LYP) method with 6-31G(d,p), 6-311++G(d,p) basis sets, using Gaussian 03W program. HOMO and LUMO energies are calculated that these energies show charge transfer occurs within the molecule.Figure optionsDownload as PowerPoint slideHighlights
► A detailed interpretation of Infrared and Raman spectra of SSMPL were reported.
► Molecular structural parameters of the geometry have been computed by DFT method with 6-31G(d,p), 6-311++G(d,p) basis sets.
► HOMO and LUMO energy gap explains the eventual charge transfer interactions taking place within the molecule.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 107, 15 April 2013, Pages 386–398