| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1330994 | 978988 | 2011 | 5 صفحه PDF | دانلود رایگان |
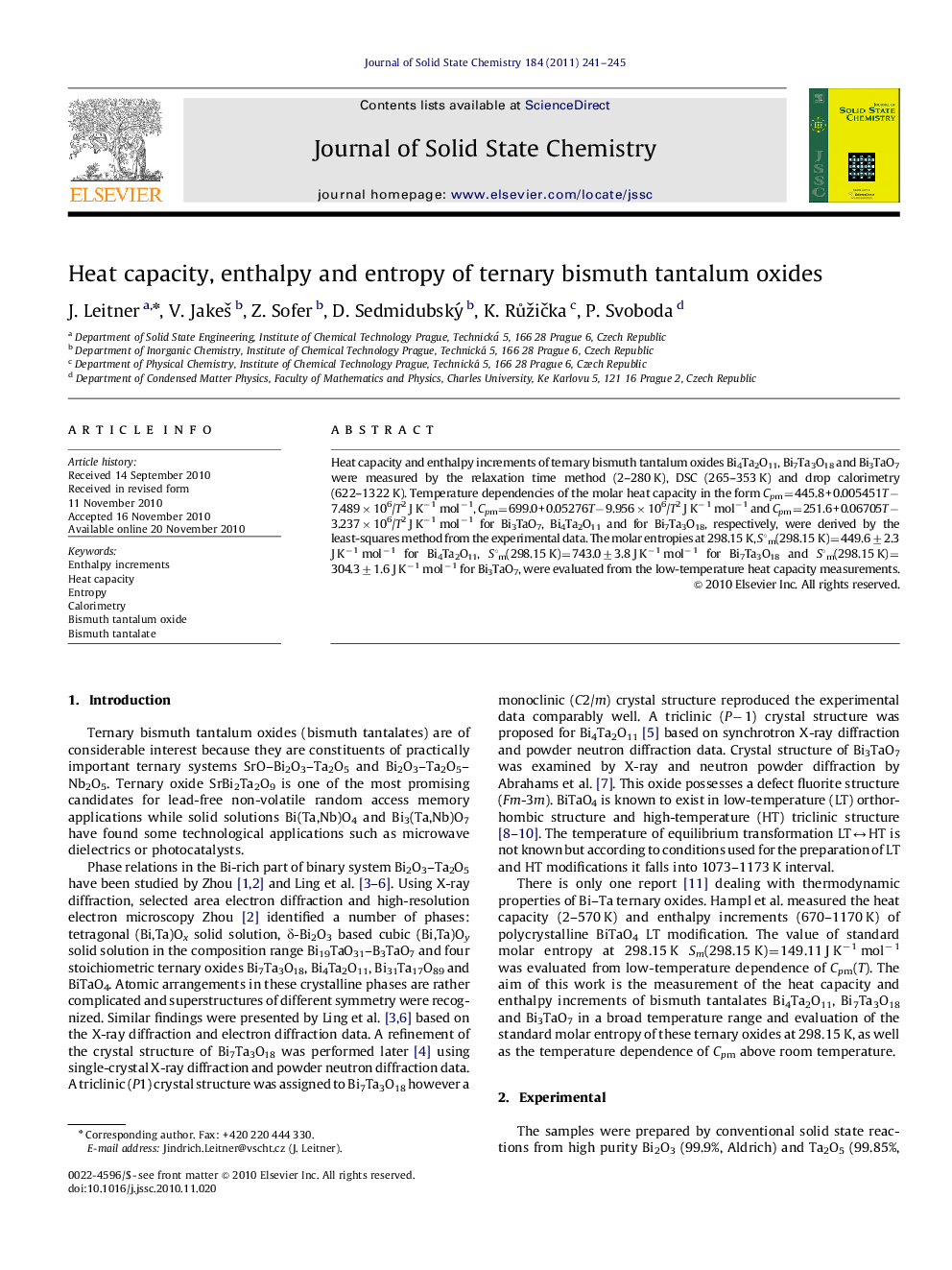
Heat capacity and enthalpy increments of ternary bismuth tantalum oxides Bi4Ta2O11, Bi7Ta3O18 and Bi3TaO7 were measured by the relaxation time method (2–280 K), DSC (265–353 K) and drop calorimetry (622–1322 K). Temperature dependencies of the molar heat capacity in the form Cpm=445.8+0.005451T−7.489×106/T2 J K−1 mol−1, Cpm=699.0+0.05276T−9.956×106/T2 J K−1 mol−1 and Cpm=251.6+0.06705T−3.237×106/T2 J K−1 mol−1 for Bi3TaO7, Bi4Ta2O11 and for Bi7Ta3O18, respectively, were derived by the least-squares method from the experimental data. The molar entropies at 298.15 K, S°m(298.15 K)=449.6±2.3 J K−1 mol−1 for Bi4Ta2O11, S°m(298.15 K)=743.0±3.8 J K−1 mol−1 for Bi7Ta3O18 and S°m(298.15 K)=304.3±1.6 J K−1 mol−1 for Bi3TaO7, were evaluated from the low-temperature heat capacity measurements.
Graphical AbstractTemperature dependence of ΔoxCpm for bismuth tantalum mixed oxides.Figure optionsDownload as PowerPoint slideResearch Highlights
► Heat capacity, enthalpy and entropy of ternary bismuth tantalum oxides Bi4Ta2O11, Bi7Ta3O18 and Bi3TaO7.
► Heat capacity by DSC calorimetry and heat-pulsed calorimetry.
► Enthalpy increments by drop calorimetry.
► Einstein–Debye model for low-temperature dependence of the heat capacity.
► Application of Neumann–Kopp rule.
Journal: Journal of Solid State Chemistry - Volume 184, Issue 2, February 2011, Pages 241–245