| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1407142 | 1501888 | 2008 | 7 صفحه PDF | دانلود رایگان |
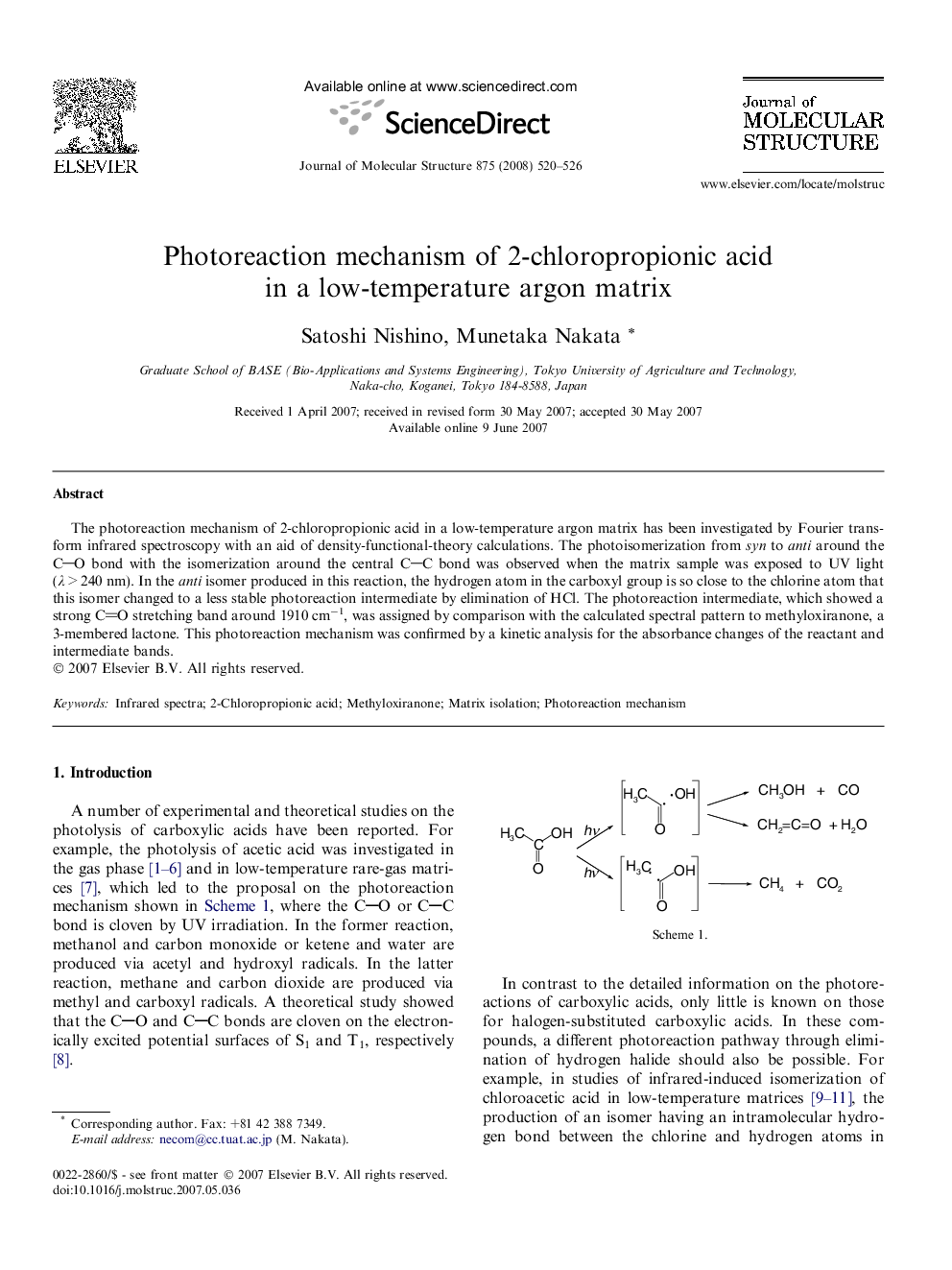
The photoreaction mechanism of 2-chloropropionic acid in a low-temperature argon matrix has been investigated by Fourier transform infrared spectroscopy with an aid of density-functional-theory calculations. The photoisomerization from syn to anti around the CO bond with the isomerization around the central CC bond was observed when the matrix sample was exposed to UV light (λ > 240 nm). In the anti isomer produced in this reaction, the hydrogen atom in the carboxyl group is so close to the chlorine atom that this isomer changed to a less stable photoreaction intermediate by elimination of HCl. The photoreaction intermediate, which showed a strong CO stretching band around 1910 cm−1, was assigned by comparison with the calculated spectral pattern to methyloxiranone, a 3-membered lactone. This photoreaction mechanism was confirmed by a kinetic analysis for the absorbance changes of the reactant and intermediate bands.
Journal: Journal of Molecular Structure - Volume 875, Issues 1–3, 17 March 2008, Pages 520–526