| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 75437 | 49115 | 2011 | 8 صفحه PDF | دانلود رایگان |
The preparation of nano-crystalline MFI zeolites (Silicalite-1 and ZSM-5) was carried out by hydrothermal synthesis in a water/surfactant/organic solvent using fumed silica and aluminum sulfate as the Si and Al source, respectively. It was confirmed that the surfactant in the solution affected the nucleation rate of the MFI zeolite. Moreover, the crystal size of the MFI zeolite decreased with increasing surfactant concentration, and nanometer-sized MFI zeolites was obtained at a surfactant concentration of 0.25–0.5 mol/L. The successful preparation of MFI zeolite nanocrystals was ascribed to the stabilization of the MFI zeolite precursors and/or crystals by the adsorbed surfactant on their surface. This method was applied to the preparation of nano-crystalline ZSM-5 zeolite. ZSM-5 zeolite with a crystal size of approximately 50 nm was obtained. The nano-crystalline ZSM-5 zeolite was well-crystallized without octahedral Al atoms in the external framework, and exhibited almost the same acidity as a reference ZSM-5 zeolite.
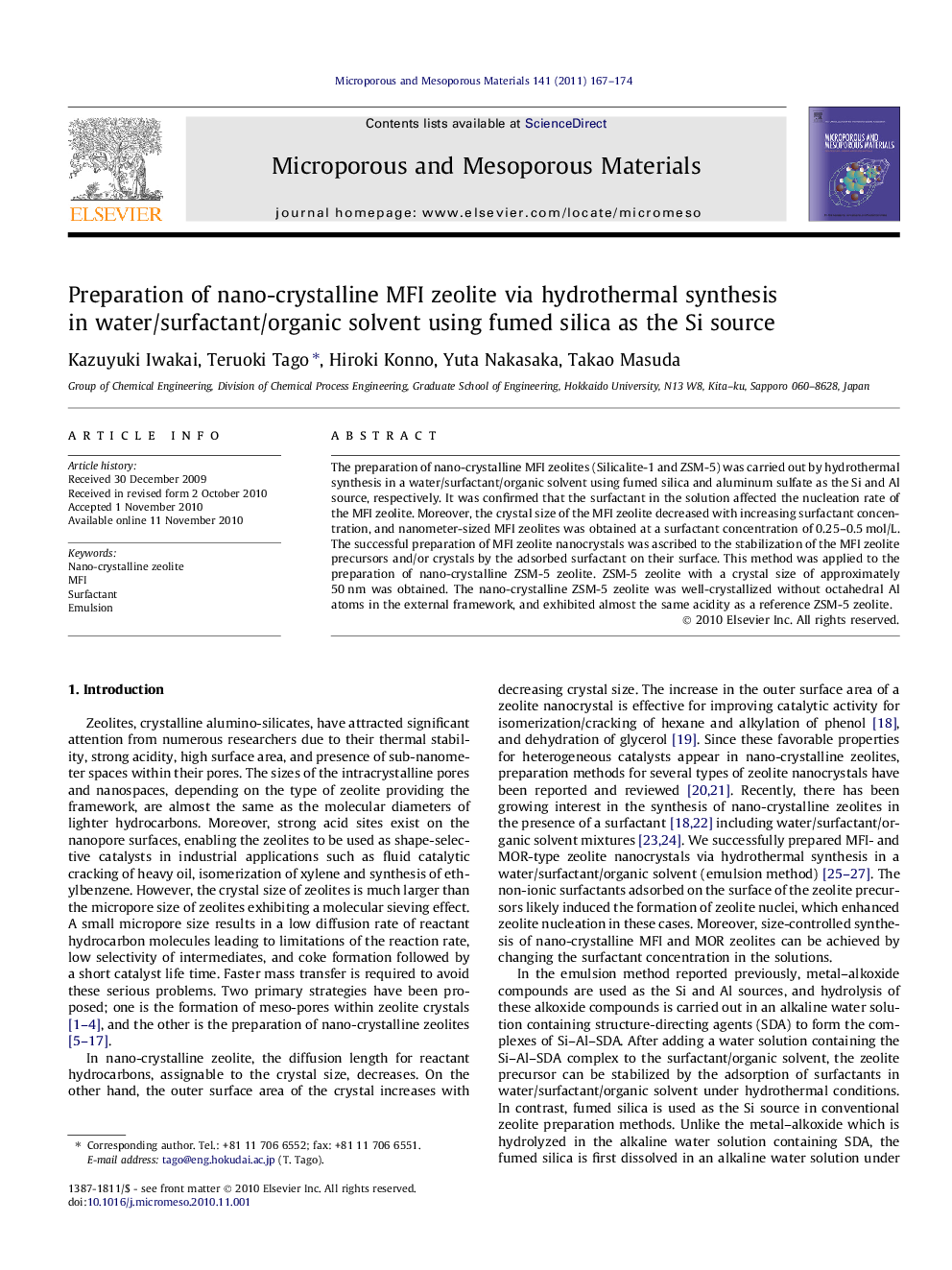
A surfactant in the synthetic solution affected a nucleation rate of zeolite, leading to preparation of MFI nanocrystals. Molar ratio of water to surfactant (W/S) can control the crystal sizes.Figure optionsDownload as PowerPoint slideResearch highlights
► The preparation of nano-crystalline MFI zeolites was carried out by hydrothermal synthesis in water/surfactant/organic solvent mixtures (emulsion method).
► The major difference between the conventional and emulsion methods was the nucleation rate of zeolite.
► The surfactant in the solution affected the nucleation rate of the MFI zeolite, leading to the formation of mono-dispersed Silicalite-1 nanocrystals.
► The crystal sizes of the MFI zeolite were controlled by the molar ratio of water to surfactant (W/S).
Journal: Microporous and Mesoporous Materials - Volume 141, Issues 1–3, May 2011, Pages 167–174