| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347955 | 980332 | 2008 | 6 صفحه PDF | دانلود رایگان |
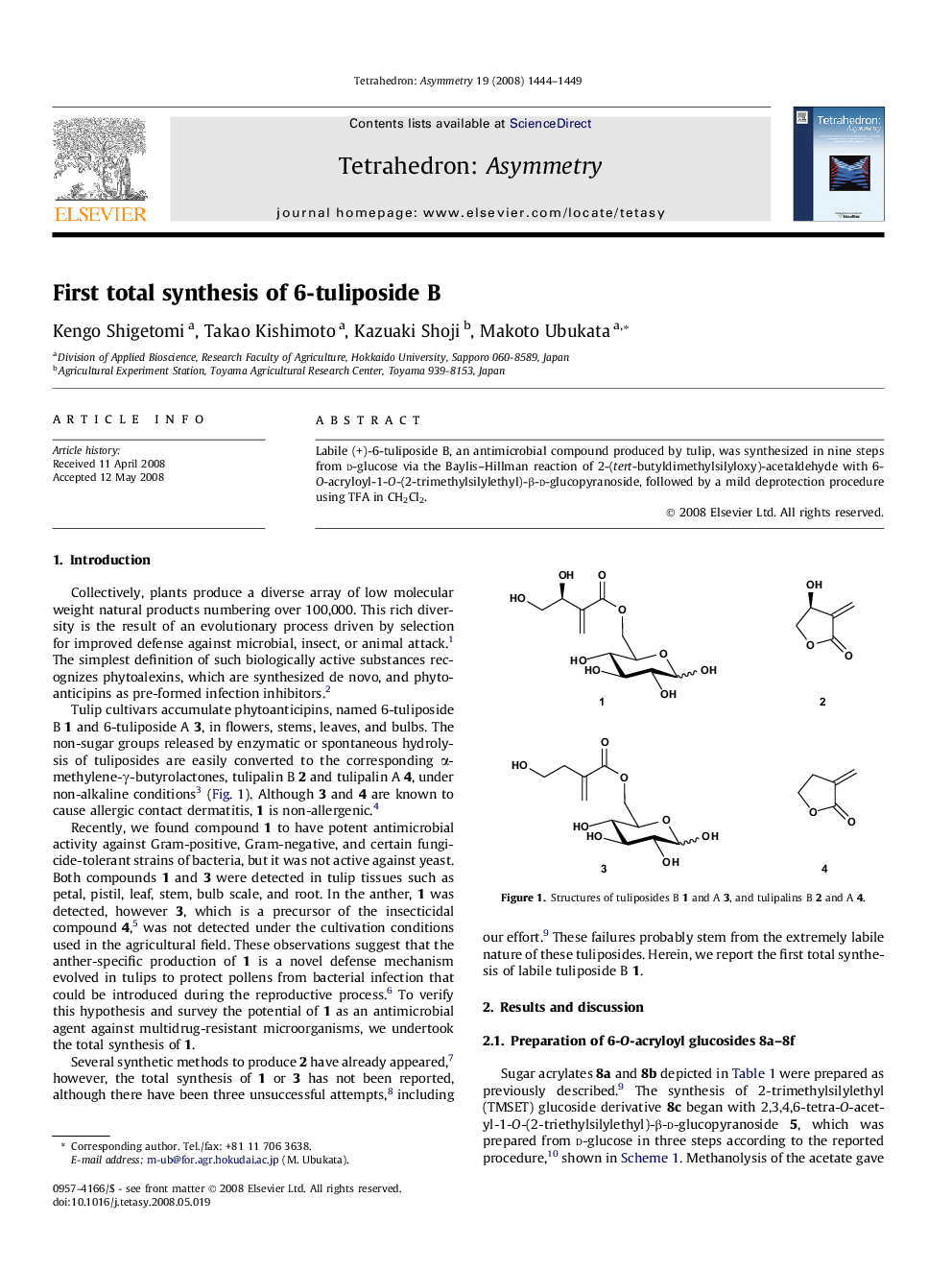
Labile (+)-6-tuliposide B, an antimicrobial compound produced by tulip, was synthesized in nine steps from d-glucose via the Baylis–Hillman reaction of 2-(tert-butyldimethylsilyloxy)-acetaldehyde with 6-O-acryloyl-1-O-(2-trimethylsilylethyl)-β-d-glucopyranoside, followed by a mild deprotection procedure using TFA in CH2Cl2.
Figure optionsDownload as PowerPoint slide
(+)-6-Tuliposide BC11H18O9[α]D = +37.7 (c 1.0, MeOH)Source of chirality: d-glucoseAbsolute configuration: (3′S)
(3′R)-epi-6-Tuliposide BC11H18O9[α]D = +30.9 (c 1.0, MeOH)Source of chirality: d-glucoseAbsolute configuration: (3′R)
1-O-(2-Trimethylsilylethyl)-6-O-[(3′S)-4′-(tert-butyldiethylsilyloxy)-3′-hydroxy-2′-methylenebutanoyl]-β-d-glucopyranosideC22H45O9Si2[α]D = −24.0 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (3′S)
1-O-(2-Trimethylsilylethyl)-6-O-[(3′R)-4′-(tert-butyldiethylsilyloxy)-3′-hydroxy-2′-methylenebutanoyl]-β-d-glucopyranosideC22H45O9Si2[α]D = −35.1 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (3′R)
(−)-Tulipalin BC5H6O3[α]D = −79.1 (c 0.25, MeOH)Source of chirality: 6-tuliposide BAbsolute configuration: (3S)
(+)-Tulipalin BC5H6O3[α]D = +79.4 (c 0.25, MeOH)Source of chirality: (3′R)-epi-6-tuliposide BAbsolute configuration: (3R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 12, 30 June 2008, Pages 1444–1449