| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349853 | 1500371 | 2009 | 13 صفحه PDF | دانلود رایگان |
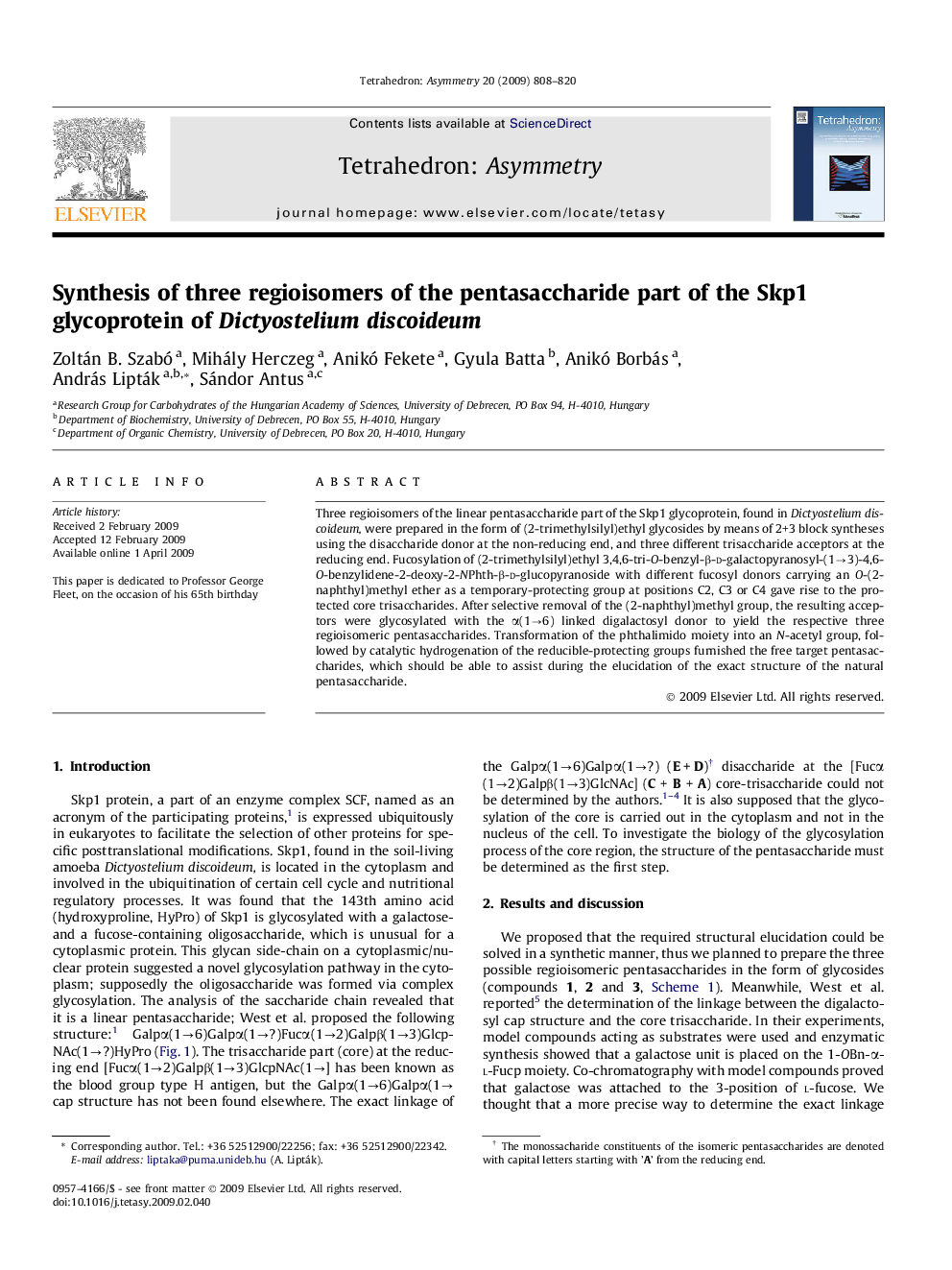
Three regioisomers of the linear pentasaccharide part of the Skp1 glycoprotein, found in Dictyostelium discoideum, were prepared in the form of (2-trimethylsilyl)ethyl glycosides by means of 2+3 block syntheses using the disaccharide donor at the non-reducing end, and three different trisaccharide acceptors at the reducing end. Fucosylation of (2-trimethylsilyl)ethyl 3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-NPhth-β-d-glucopyranoside with different fucosyl donors carrying an O-(2-naphthyl)methyl ether as a temporary-protecting group at positions C2, C3 or C4 gave rise to the protected core trisaccharides. After selective removal of the (2-naphthyl)methyl group, the resulting acceptors were glycosylated with the α(1→6) linked digalactosyl donor to yield the respective three regioisomeric pentasaccharides. Transformation of the phthalimido moiety into an N-acetyl group, followed by catalytic hydrogenation of the reducible-protecting groups furnished the free target pentasaccharides, which should be able to assist during the elucidation of the exact structure of the natural pentasaccharide.
Figure optionsDownload as PowerPoint slide
(2-Trimethylsilyl)ethyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC55H61NO13Si[α]D = −6.1 (c 0.16, CHCl3)
(2-Trimethylsilyl)ethyl 3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC53H59NO12Si[α]D = −48.5 (c 0.24, CHCl3)
(2-Trimethylsilyl)ethyl 3,4-di-O-benzyl-2-O-(2-naphthyl)methyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC84H89NO16Si[α]D = −37.7 (c 0.13, CHCl3)
(2-Trimethylsilyl)ethyl 3,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC73H81NO16Si[α]D = −47.4 (c 0.15, CHCl3)
(2-Trimethylsilyl)ethyl 2,4-di-O-benzyl-3-O-(2-naphthyl)methyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC84H89NO16Si[α]D = −33.8 (c 0.21, CHCl3)
(2-Trimethylsilyl)ethyl 2,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC73H81NO16Si[α]D = −61.0 (c 0.12, CHCl3)
(2-Trimethylsilyl)ethyl-2,3-di-O-benzyl-4-O-(2-naphthyl)methyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC84H89NO16Si[α]D = −40.5 (c 0.22, CHCl3)
(2-Trimethylsilyl)ethyl 2,3-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC73H81NO16Si[α]D = −34.2 (c 0.16, CHCl3)
Phenyl 3,4-O-isopropylidene-6-O-(methoxydimethyl)methyl-2-O-(2-naphthyl)methyl-1-thio-β-d-galactopyranosideC30H36O6S[α]D = +2.7 (c 1.01, CHCl3)
Phenyl 3,4-O-isopropylidene-2-O-(2-naphthyl)methyl-1-thio-β-d-galactopyranosideC26H28O5S[α]D = +12.8 (c 1.03, CHCl3)
Phenyl 2,3,4,6-tetra-O-benzyl-α/β-d-galactopyranosyl-(1→6)-3,4-O-isopropylidene-2-O-(2-naphthyl)methyl-1-thio-β-d-galactopyranosideC60H62O10S[α]D = +26.7 (c 1.05, CHCl3)
Phenyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-3,4-di-O-acetyl-2-O-(2-naphthyl)methyl-1-thio-β-d-galactopyranosideC61H62O12S[α]D = +28.2 (c 0.99, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-3,4-di-O-acetyl-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→2)-3,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC128H137NO28Si[α]D = +64.8 (c 0.13, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-3,4-di-O-acetyl-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→3)-2,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC128H137NO28Si[α]D = −23.6 (c 0.12, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-3,4-di-O-acetyl-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→4)-2,3-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranosideC128H137NO28Si[α]D = −4.3 (c 0.14, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→2)-3,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-2-acetamido-4,6-O-benzylidene-2-deoxy-β-d-glucopyranosideC118H133NO25Si[α]D = +6.8 (c 0.13, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→3)-2,4-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-2-acetamido-4,6-O-benzylidene-2-deoxy-β-d-glucopyranosideC118H133NO25Si[α]D = −11.5 (c 0.15, CHCl3)
(2-Trimethylsilyl)ethyl 2,3,4,6-tetra-O-benzyl-α-d-galactopyranosyl-(1→6)-2-O-(2-naphthyl)methyl-α-d-galactopyranosyl-(1→4)-2,3-di-O-benzyl-α-l-fucopyranosyl-(1→2)-3,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-2-acetamido-4,6-O-benzylidene-2-deoxy-β-d-glucopyranosideC118H133NO25Si[α]D = +5.8 (c 0.12, CHCl3)
(2-Trimethylsilyl)ethyl α-d-galactopyranosyl-(1→6)-α-d-galactopyranosyl-(1→2)-α-l-fucopyranosyl-(1→2)-β-d-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranosideC37H67NO25Si[α]D = +29.7 (c 0.14, MeOH)
(2-Trimethylsilyl)ethyl α-d-galactopyranosyl-(1→6)-α-[-galactopyranosyl-(1→3)-α-l-fucopyranosyl-(1→2)-β-d-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranosideC37H67NO25Si[α]D = +21.1 (c 0.18, MeOH)
(2-Trimethylsilyl)ethyl α-d-galactopyranosyl-(1→6)-α-d-galactopyranosyl-(1→4)-α-l-fucopyranosyl-(1→2)-β-d-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranosideC37H67NO25Si[α]D = +22.6 (c 0.22, MeOH)
Journal: Tetrahedron: Asymmetry - Volume 20, Issues 6–8, 7 May 2009, Pages 808–820