| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1523859 | 995331 | 2012 | 9 صفحه PDF | دانلود رایگان |
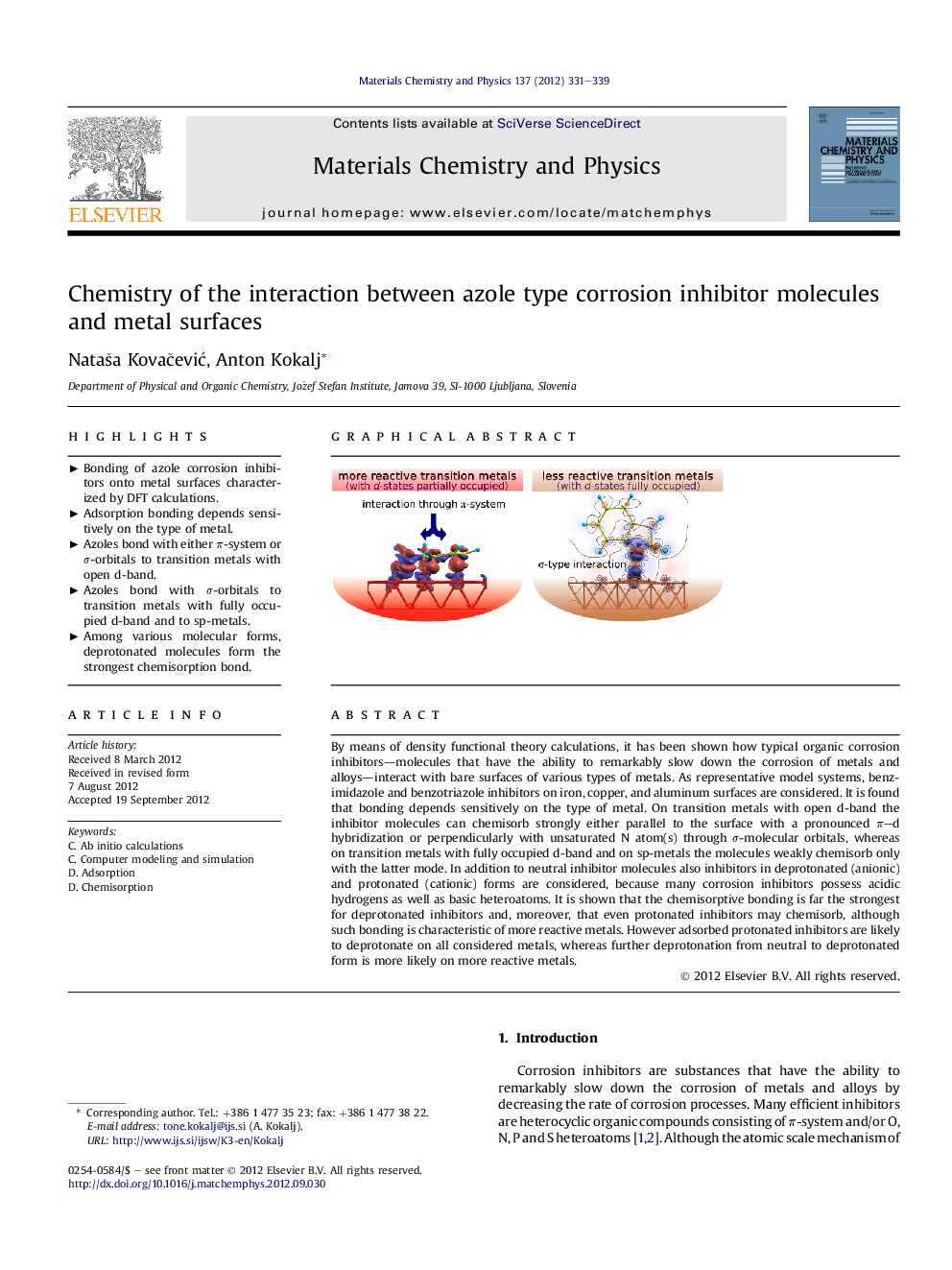
By means of density functional theory calculations, it has been shown how typical organic corrosion inhibitors—molecules that have the ability to remarkably slow down the corrosion of metals and alloys—interact with bare surfaces of various types of metals. As representative model systems, benzimidazole and benzotriazole inhibitors on iron, copper, and aluminum surfaces are considered. It is found that bonding depends sensitively on the type of metal. On transition metals with open d-band the inhibitor molecules can chemisorb strongly either parallel to the surface with a pronounced π–d hybridization or perpendicularly with unsaturated N atom(s) through σ-molecular orbitals, whereas on transition metals with fully occupied d-band and on sp-metals the molecules weakly chemisorb only with the latter mode. In addition to neutral inhibitor molecules also inhibitors in deprotonated (anionic) and protonated (cationic) forms are considered, because many corrosion inhibitors possess acidic hydrogens as well as basic heteroatoms. It is shown that the chemisorptive bonding is far the strongest for deprotonated inhibitors and, moreover, that even protonated inhibitors may chemisorb, although such bonding is characteristic of more reactive metals. However adsorbed protonated inhibitors are likely to deprotonate on all considered metals, whereas further deprotonation from neutral to deprotonated form is more likely on more reactive metals.
Figure optionsDownload as PowerPoint slideHighlights
► Bonding of azole corrosion inhibitors onto metal surfaces characterized by DFT calculations.
► Adsorption bonding depends sensitively on the type of metal.
► Azoles bond with either π-system or σ-orbitals to transition metals with open d-band.
► Azoles bond with σ-orbitals to transition metals with fully occupied d-band and to sp-metals.
► Among various molecular forms, deprotonated molecules form the strongest chemisorption bond.
Journal: Materials Chemistry and Physics - Volume 137, Issue 1, 15 November 2012, Pages 331–339