| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1304861 | 974855 | 2007 | 5 صفحه PDF | دانلود رایگان |
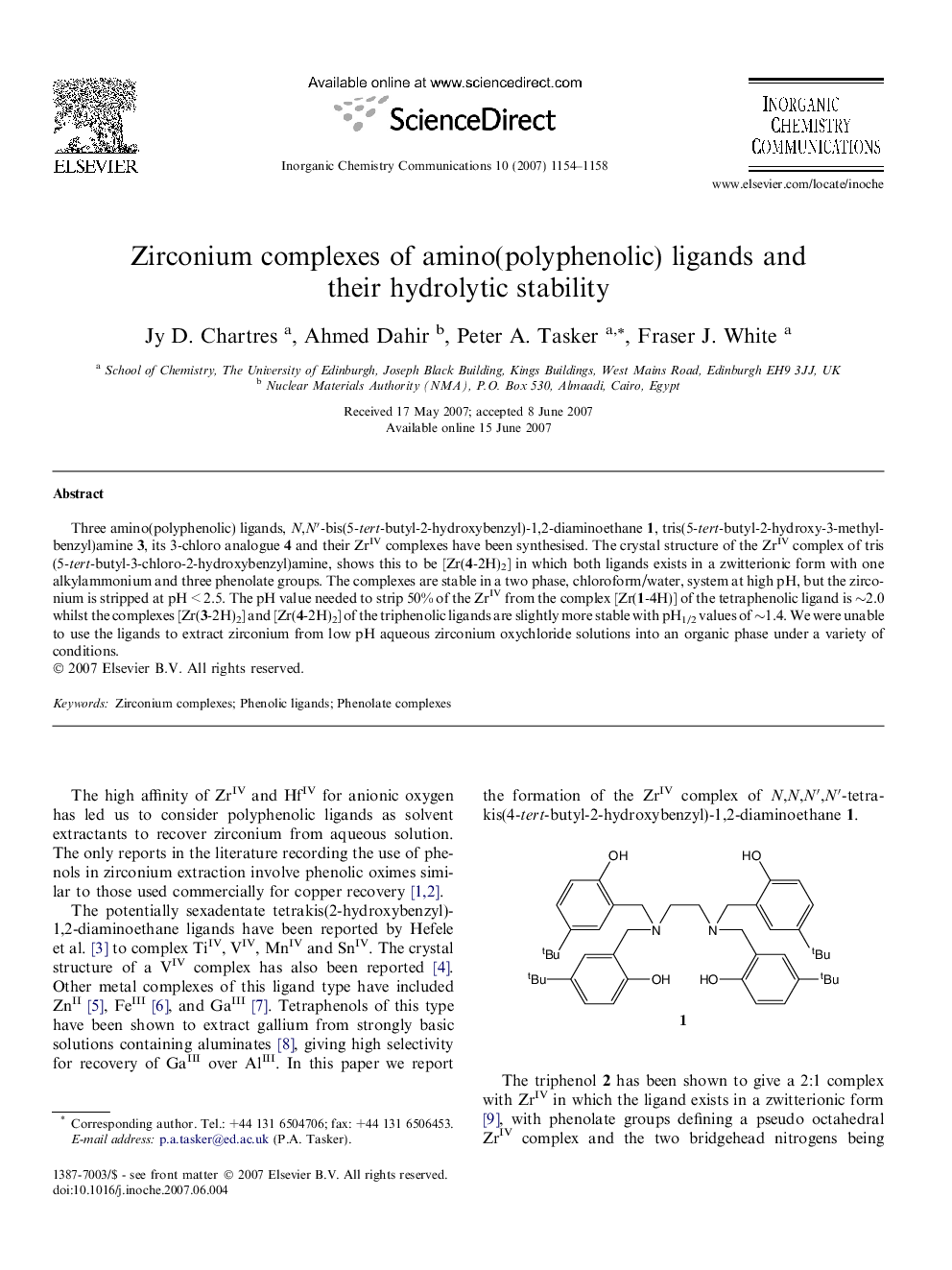
Three amino(polyphenolic) ligands, N,N′-bis(5-tert-butyl-2-hydroxybenzyl)-1,2-diaminoethane 1, tris(5-tert-butyl-2-hydroxy-3-methylbenzyl)amine 3, its 3-chloro analogue 4 and their ZrIV complexes have been synthesised. The crystal structure of the ZrIV complex of tris(5-tert-butyl-3-chloro-2-hydroxybenzyl)amine, shows this to be [Zr(4-2H)2] in which both ligands exists in a zwitterionic form with one alkylammonium and three phenolate groups. The complexes are stable in a two phase, chloroform/water, system at high pH, but the zirconium is stripped at pH < 2.5. The pH value needed to strip 50% of the ZrIV from the complex [Zr(1-4H)] of the tetraphenolic ligand is ∼2.0 whilst the complexes [Zr(3-2H)2] and [Zr(4-2H)2] of the triphenolic ligands are slightly more stable with pH1/2 values of ∼1.4. We were unable to use the ligands to extract zirconium from low pH aqueous zirconium oxychloride solutions into an organic phase under a variety of conditions.
Three amino(polyphenolic) ligands and their ZrIV complexes have been synthesised. The crystal structure of the ZrIV complex of tris(5-tert-butyl-3-chloro-2-hydroxybenzyl)amine, shows this to be [Zr(L-2H)2] in which both ligands exists in a zwitterionic form. The complexes are stable in a two phase system at high pH, but the zirconium is stripped at pH < 2.5.Figure optionsDownload as PowerPoint slide
Journal: Inorganic Chemistry Communications - Volume 10, Issue 10, October 2007, Pages 1154–1158