| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343570 | 980010 | 2015 | 12 صفحه PDF | دانلود رایگان |
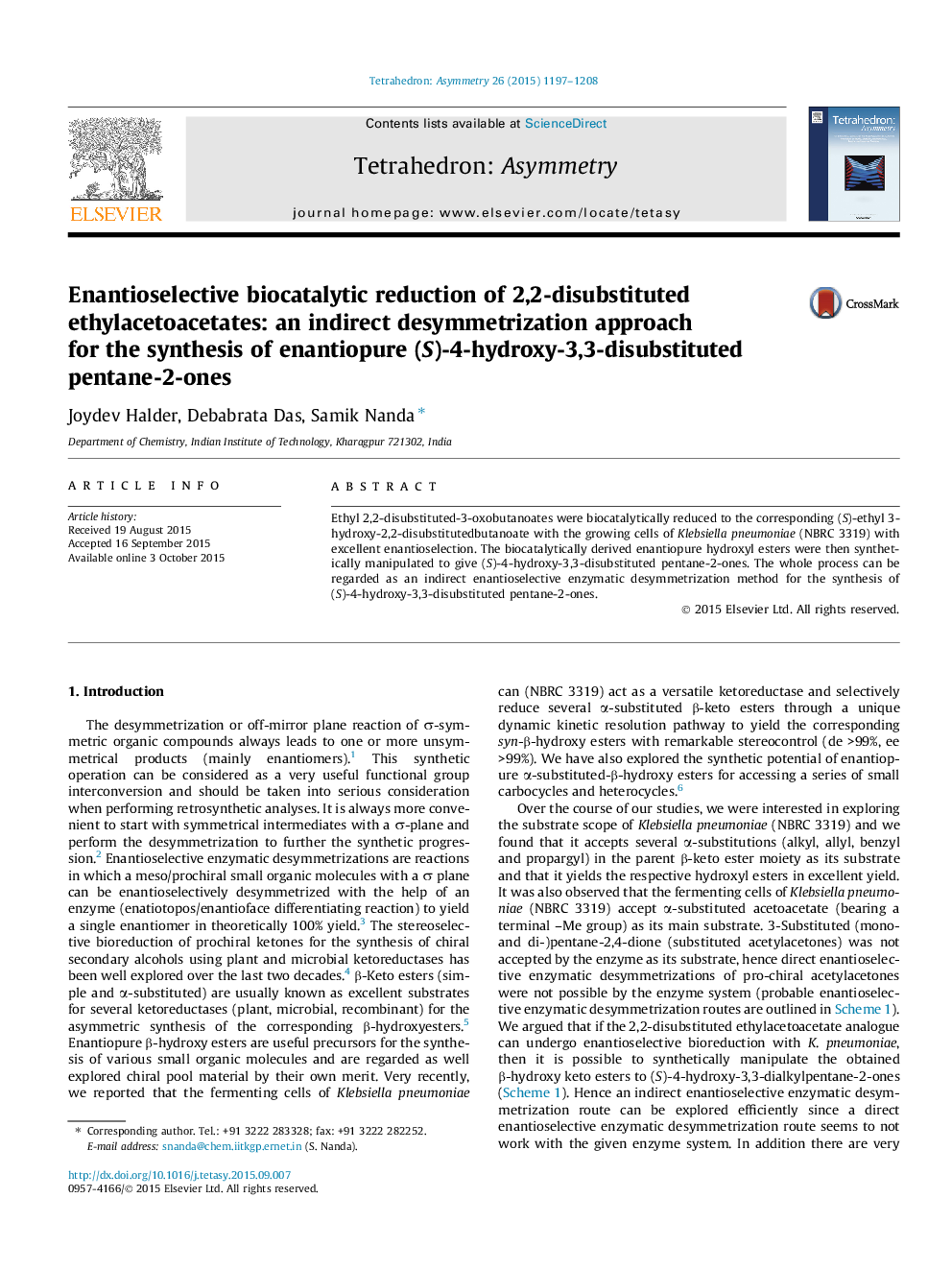
Ethyl 2,2-disubstituted-3-oxobutanoates were biocatalytically reduced to the corresponding (S)-ethyl 3-hydroxy-2,2-disubstitutedbutanoate with the growing cells of Klebsiella pneumoniae (NBRC 3319) with excellent enantioselection. The biocatalytically derived enantiopure hydroxyl esters were then synthetically manipulated to give (S)-4-hydroxy-3,3-disubstituted pentane-2-ones. The whole process can be regarded as an indirect enantioselective enzymatic desymmetrization method for the synthesis of (S)-4-hydroxy-3,3-disubstituted pentane-2-ones.
Figure optionsDownload as PowerPoint slide
(S)-Ethyl 3-hydroxy-2,2-dimethylbutanoateC8H16O3[α]D28 = +9.8 (c 1.2, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2,2-diethyl-3-hydroxybutanoateC10H20O3[α]D28 = +11.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-(1-hydroxyethyl)-2-propylpentanoateC12H24O3[α]D28 = +4.5 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-butyl-2-(1-hydroxyethyl)hexanoateC14H28O3[α]D28 = +12.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-(1-hydroxyethyl)-2-isobutyl-4-methylpentanoateC14H28O3[α]D28 = +10.8 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-(1-hydroxyethyl)-2-pentylheptanoateC16H32O3[α]D28 = +16.4 (c 1.2, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-hexyl-2-(1-hydroxyethyl)octanoateC18H36O3[α]D28 = +15.8 (c 0.7, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-allyl-2-(1-hydroxyethyl)pent-4-enoateC12H20O3[α]D28 = +23.3 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-(but-3-enyl)-2-(1-hydroxyethyl)hex-5-enoateC14H24O3[α]D28 = +19.2 (c 0.6, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2,2-dibenzyl-3-hydroxybutanoateC20H24O3[α]D28 = +33.2 (c 0.5, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 3-hydroxy-2,2-bis(4-methoxybenzyl)butanoateC22H28O5[α]D28 = +28.6 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 2-(1-hydroxyethyl)-2-(prop-2-ynyl)pent-4-ynoateC12H16O3[α]D28 = +15.6 (c 0.4, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 1-(1-hydroxyethyl)cyclopent-3-enecarboxylateC10H16O3[α]D28 = +38.2 (c 0.5, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 1-(1-hydroxyethyl)cyclopentanecarboxylateC10H18O3[α]D28 = +32.6 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S,Z)-Ethyl 1-(1-hydroxyethyl)cyclohept-4-enecarboxylatC12H20O3[α]D28 = +22.7 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 1-(1-hydroxyethyl)cycloheptanecarboxylateC12H22O3[α]D28 = +29.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: See >99%
(S)-Ethyl 3-(tert-butyldimethylsilyloxy)-2,2-diethylbutanoateC16H34O3Si[α]D28 = +30.2 (c 1.1, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-Ethyl 2-allyl-2-(1-(tert-butyldimethylsilyloxy)ethyl)pent-4-enoateC18H34O3Si[α]D28 = +18.9 (c 0.6, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-Ethyl 3-(tert-butyldimethylsilyloxy)-2,2-bis(4-methoxybenzyl)butanoateC28H42O5Si[α]D28 = +44.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-Ethyl 1-(1-(tert-butyldimethylsilyloxy)ethyl)cyclopent-3-enecarboxylateC16H30O3Si[α]D28 = +23.4 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-4-(tert-Butyldimethylsilyloxy)-3,3-diethylpentan-2-oneC15H32O2Si[α]D28 = +11.4 (c 0.3, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-3-Allyl-3-(1-(tert-butyldimethylsilyloxy)ethyl)hex-5-en-2-oneC17H32O2Si[α]D28 = +5.8 (c 0.4, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-4-(tert-Butyldimethylsilyloxy)-3,3-bis(4-methoxybenzyl)pentan-2-oneC27H40O4Si[α]D28 = +22.6 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-1-(1-(1-(tert-Butyldimethylsilyloxy)ethyl)cyclopent-3-enyl)ethanoneC15H28O2Si[α]D28 = +39.2 (c 0.6, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-3,3-Diethyl-4-hydroxypentan-2-oneC9H18O2[α]D28 = +37.0 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-3-Allyl-3-(1-hydroxyethyl)hex-5-en-2-oneC11H18O2[α]D28 = +28.2 (c 0.5, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-4-Hydroxy-3,3-bis(4-methoxybenzyl)pentan-2-oneC21H26O4[α]D28 = +44.5 (c 1.1, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-1-(1-(1-Hydroxyethyl)cyclopent-3-enyl)ethanoneC9H14O2[α]D28 = +29.2 (c 0.7, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)ee >99%
(S)-2-((S)-1-(tert-Butyldimethylsilyloxy)ethyl)-2-methylpent-4-en-1-olC14H30O2Si[α]D28 = +6.8 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S,S)ee = 99%
(R)-2-((S)-1-(tert-Butyldimethylsilyloxy)ethyl)-2-methylpent-4-en-1-olC14H30O2Si[α]D28 = +11.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (R,S)ee = 99%
(2S,3S)-2-Benzyl-3-(tert-butyldimethylsilyloxy)-2-methylbutan-1-olC18H32O2Si[α]D28 = +22.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (2S,3S)ee = 99%
(2R,3S)-2-Benzyl-3-(tert-butyldimethylsilyloxy)-2-methylbutan-1-olC18H32O2Si[α]D28 = +4.8 (c 1.2, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (2R,3S)ee = 99%
(R)-3-((S)-1-(tert-Butyldimethylsilyloxy)ethyl)-3-methylhex-5-en-2-oneC15H30O2Si[α]D28 = +28.5 (c 0.5, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (R,S)ee = 99%
(S)-3-((S)-1-(tert-butyldimethylsilyloxy)ethyl)-3-methylhex-5-en-2-oneC15H30O2Si[α]D28 = +8.6 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S,S)ee = 99%
(3R,4S)-3-Benzyl-4-(tert-butyldimethylsilyloxy)-3-methylpentan-2-oneC19H32O2Si[α]D28 = +44.2 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (3R,4S)ee = 99%
(3S,4S)-3-Benzyl-4-(tert-butyldimethylsilyloxy)-3-methylpentan-2-oneC19H32O2Si[α]D28 = +22.6 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (3S,4S)ee = 99%
(R)-3-((S)-1-Hydroxyethyl)-3-methylhex-5-en-2-oneC9H16O2[α]D28 = +24.6 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (R,S)ee = 99%
(S)-3-((S)-1-Hydroxyethyl)-3-methylhex-5-en-2-oneC9H16O2[α]D28 = +16.7 (c 0.8, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S,S)ee = 99%
(3R,4S)-3-Benzyl-4-hydroxy-3-methylpentan-2-oneC13H18O2[α]D28 = +12.6 (c 0.6, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (3R,4S)ee = 99%
(3S,4S)-3-Benzyl-4-hydroxy-3-methylpentan-2-oneC13H18O2[α]D28 = +5.8 (c 0.4, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (3S,4S)ee = 99%
Journal: Tetrahedron: Asymmetry - Volume 26, Issue 20, 1 November 2015, Pages 1197–1208