| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343728 | 1500342 | 2015 | 10 صفحه PDF | دانلود رایگان |
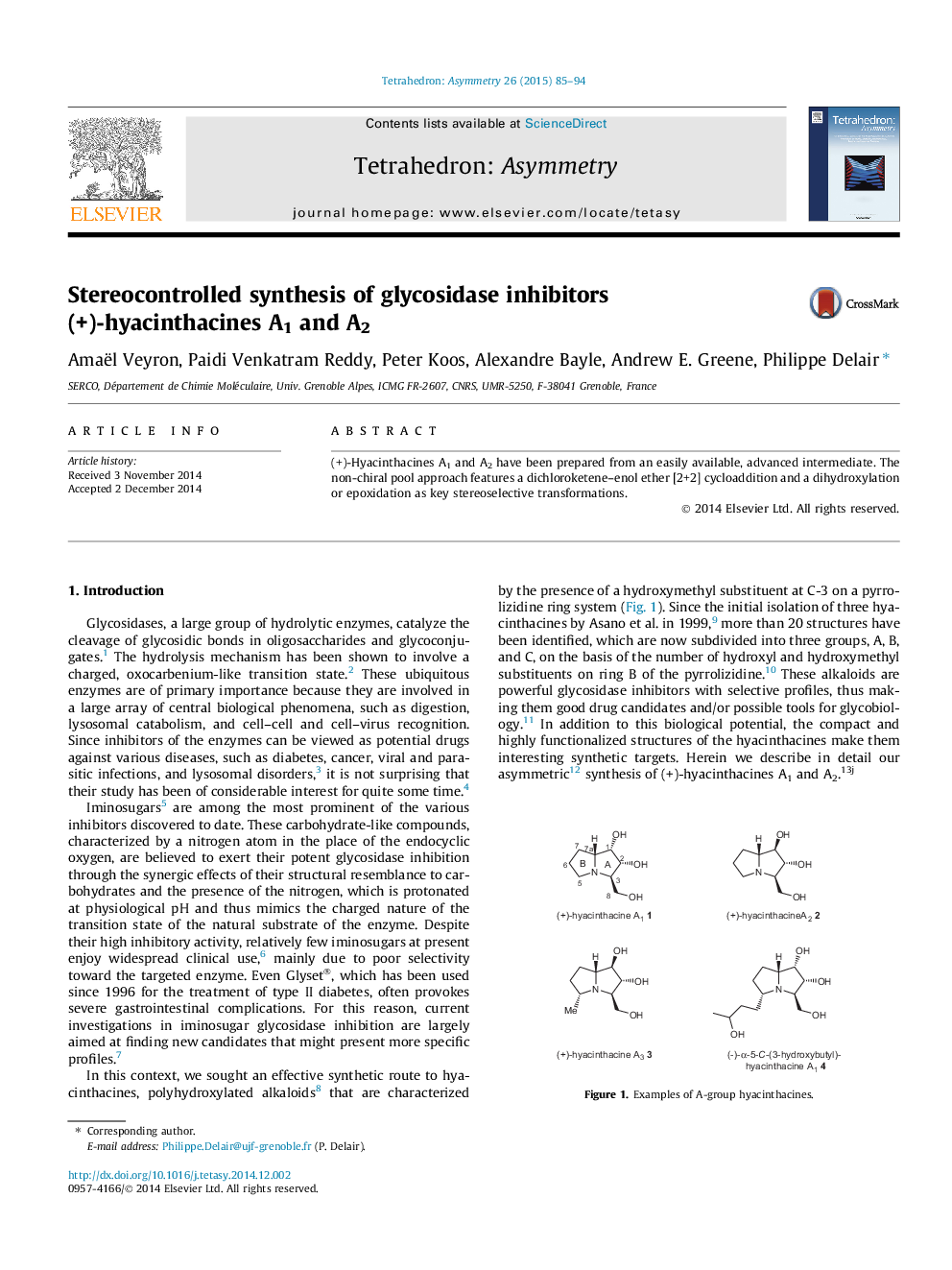
(+)-Hyacinthacines A1 and A2 have been prepared from an easily available, advanced intermediate. The non-chiral pool approach features a dichloroketene–enol ether [2+2] cycloaddition and a dihydroxylation or epoxidation as key stereoselective transformations.
Figure optionsDownload as PowerPoint slide
(+)-Hyacinthacine A1C8H15NO3[α]D21 = +43.9 (c 0.3, H2O)Source of chirality: (S)-(−)-StericolAbsolute configuration: (1S,2R,3R,7aR)
(+)-Hyacinthacine A2C8H15NO3[α]D23 = +12.0 (c 0.45, H2O)Source of chirality: (S)-(−)-StericolAbsolute configuration: (1R,2R,3R,7aR)
(2R,3R)-tert-Butyl 2-allyl-5-oxo-3-[(S)-1-(2,4,6-triisopropylphenyl)ethoxy]-pyrrolidine-1-carboxylateC29H45NO4[α]D21 = −98.4 (c 0.6, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (2R,3R)
(1R,3S,7aR)-3-{[Dimethyl(phenyl)silyl]methyl}-hexahydro-1H-pyrrolizin-1-olC16H25NOSi[α]D21 = −45.0 (c 0.91, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (1R,3S,7aR)
(5S,6R,7S,7aR)-6,7-Dihydroxy-5-((dimethyl(phenyl)silyl)methyl)-hexahydropyrrolizin-3-oneC16H23NO3Si[α]D21 = −58.7 (c 1.1, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,6R,7S,7aR)
(5S,7R,7aR)-5-((Dimethyl(phenyl)silyl)methyl)-7-((S)-1-(2,4,6-triisopropylphenyl)ethoxy)-hexahydro-1H-pyrrolizin-3-oneC33H49NO2Si[α]D21 = −82.9 (c 1.4, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,7R,7aR)
(5S,7R,7aR)-5-((Dimethyl(phenyl)silyl)methyl)-7-hydroxy-hexahydro-1H-pyrrolizin-3-oneC16H23NO2Si[α]D21 = −73.4 (c 1.6, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,7R,7aR)
(5S,7aR)-5-((Dimethyl(phenyl)silyl)methyl)-1,2,5,7a-tetrahydropyrrolizin-3-oneC16H21NOSi[α]D21 = −177.2 (c 0.7, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,7aR)
(5R,6R,7S,7aR)-6,7-Dihydroxy-5-(hydroxymethyl)hexahydropyrrolizin-3-oneC8H13NO4[α]D21 = −49.9 (c 0.7, CH3OH)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,6R,7S,7aR)
(1aR,2S,6aR,6bS)-2-((Dimethyl(phenyl)silylmethyl)-hexahydro-oxireno[2,3-a]pyrrolizin-4-oneC16H21NO2[α]D21 = −95.6 (c 1.2, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (1aR,2S,6aR,6bS)
(5S,6R,7R,7aR)-6,7-Dihydroxy-5-((dimethyl(phenyl)silyl)methyl)-hexahydropyrrolizin-3-oneC16H23NO3Si[α]D21 = −52.1 (c 0.7, CHCl3)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5S,6R,7R,7aR)
(5R,6R,7R,7aR)-6,7-Dihydroxy-5-(hydroxymethyl)hexahydropyrrolizin-3-oneC8H13NO4[α]D23 = −46.3 (c 1.2, MeOH)Source of chirality: (S)-(−)-StericolAbsolute configuration: (5R,6R,7R,7aR)
Journal: Tetrahedron: Asymmetry - Volume 26, Issues 2–3, 15 February 2015, Pages 85–94