| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343734 | 1500342 | 2015 | 5 صفحه PDF | دانلود رایگان |
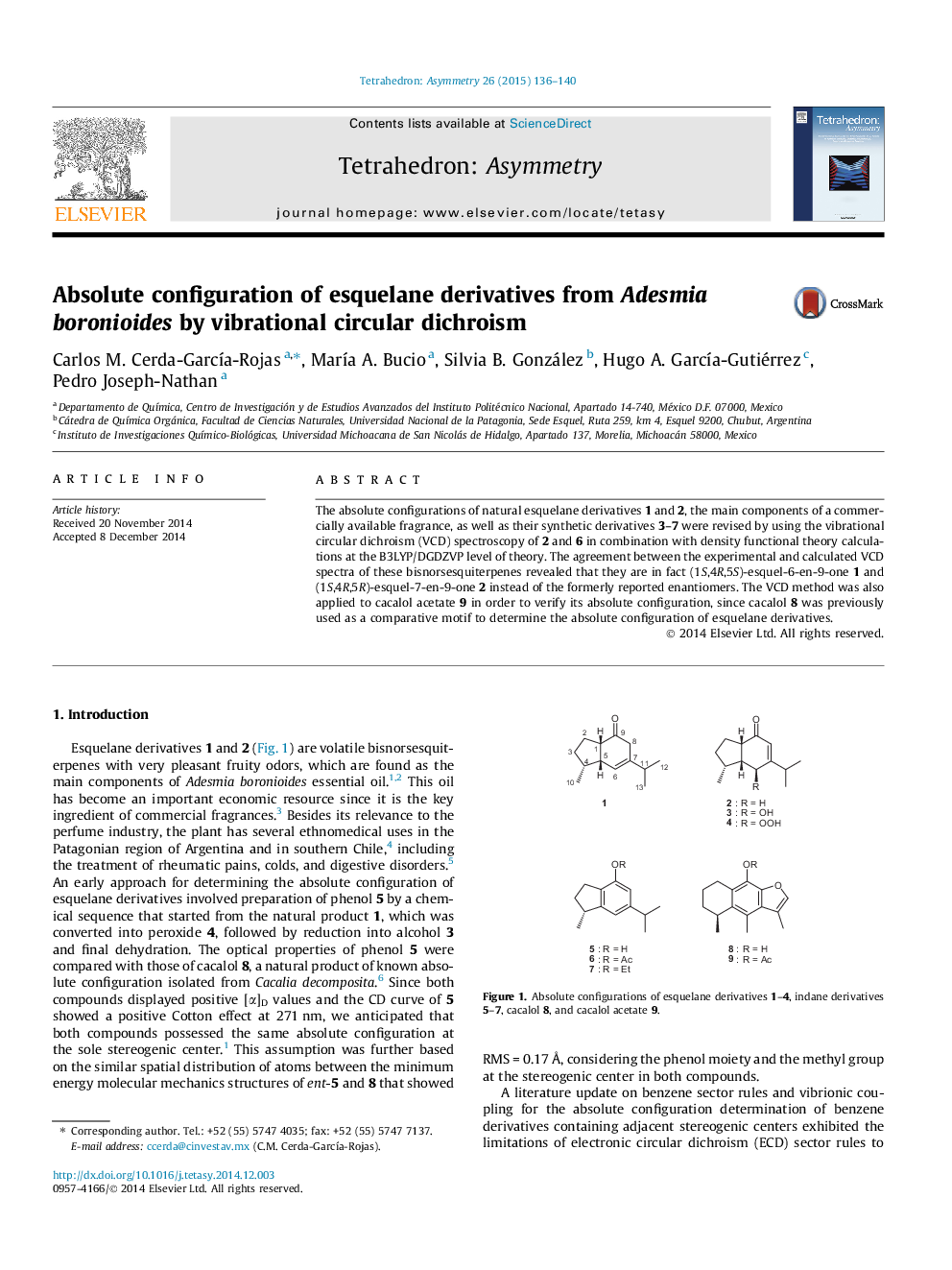
The absolute configurations of natural esquelane derivatives 1 and 2, the main components of a commercially available fragrance, as well as their synthetic derivatives 3–7 were revised by using the vibrational circular dichroism (VCD) spectroscopy of 2 and 6 in combination with density functional theory calculations at the B3LYP/DGDZVP level of theory. The agreement between the experimental and calculated VCD spectra of these bisnorsesquiterpenes revealed that they are in fact (1S,4R,5S)-esquel-6-en-9-one 1 and (1S,4R,5R)-esquel-7-en-9-one 2 instead of the formerly reported enantiomers. The VCD method was also applied to cacalol acetate 9 in order to verify its absolute configuration, since cacalol 8 was previously used as a comparative motif to determine the absolute configuration of esquelane derivatives.
Figure optionsDownload as PowerPoint slide
(1S,4R,5S)-Esquel-6-en-9-oneC13H20O100% ee[α]D20 = +18 (c 2.0, CHCl3)Source of chirality: Adesmia boronioides essential oilAbsolute configuration: (1S,4R,5S)
(1S,4R,5R)-1,2,3,4,5,6-Hexahydro-4-methyl-7-(1-methylethyl)-9H-inden-9-oneC13H20O100% ee[α]D20 = +18 (c 2.0, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1S,4R,5R)
(1S,4R,5R,6S)-6-Hydroxyesquel-7-en-9-oneC13H20O2100% ee[α]D20 = −2 (c 2.0, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1S,4R,5R,6S)
(1S,4R,5R,6S)-6-Hydroperoxyesquel-7-en-9-oneC13H20O3100% ee[α]D20 = +2 (c 3.0, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1S,4R,5R,6S)
(1R)-2,3-Dihydro-1-methyl-6-(1-methylethyl)-1H-inden-4-olC13H18O100% ee[α]D20 = +1 (c 5.0, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1R)
(1R)-2,3-Dihydro-1-methyl-6-(1-methylethyl)-1H-inden-4-ol 4-acetateC15H20O2100% ee[α]D20 = +15 (c 0.12, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1S,4R,5R)
(1R)-4-Ethoxy-2,3-dihydro-1-methyl-6-(1-methylethyl)-1H-indeneC15H22O100% ee[α]D20 = +2 (c 1.2, CHCl3)Source of chirality: (1S,4R,5S)-esquel-6-en-9-oneAbsolute configuration: (1R)
(5S)-5,6,7,8-Tetrahydro-3,4,5-trimethyl-naphtho[2,3-b]furan-9-olC15H18O2100% ee[α]D20 = +10 (c 0.12, CHCl3)Source of chirality: Cacalia decompositaAbsolute configuration: (5S)
(5S)-5,6,7,8-Tetrahydro-3,4,5-trimethyl-naphtho[2,3-b]furan-9-ol 9-acetateC17H20O3100% ee[α]D20 = −9 (c 0.12, CHCl3)Source of chirality: (5S)-5,6,7,8-tetrahydro-3,4-dimethylnaphtho[2,3-b]furan-9-olAbsolute configuration: (5S)
Journal: Tetrahedron: Asymmetry - Volume 26, Issues 2–3, 15 February 2015, Pages 136–140