| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343932 | 980046 | 2013 | 4 صفحه PDF | دانلود رایگان |
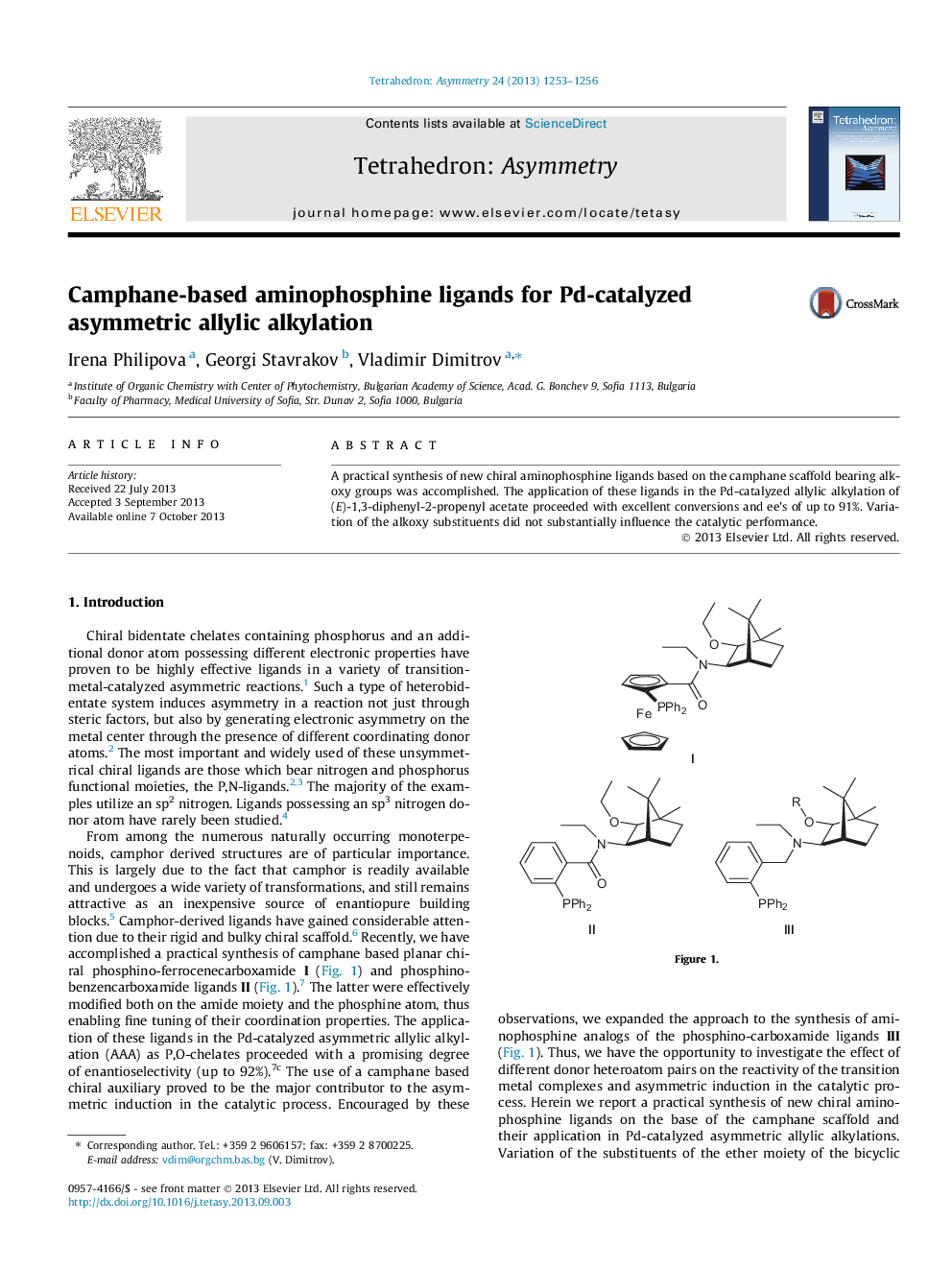
A practical synthesis of new chiral aminophosphine ligands based on the camphane scaffold bearing alkoxy groups was accomplished. The application of these ligands in the Pd-catalyzed allylic alkylation of (E)-1,3-diphenyl-2-propenyl acetate proceeded with excellent conversions and ee’s of up to 91%. Variation of the alkoxy substituents did not substantially influence the catalytic performance.
Figure optionsDownload as PowerPoint slide
(1S,2R,3S,4R)-N-(2-(Diphenylphosphino)benzyl)-3-ethoxy-N-ethyl-4,7,7-trimethylbicyclo[2.2.1]heptan-2-amineC33H42NOPDe >99% (NMR)[α]D20=+54.9 (c 0.85, CHCl3)Source of chirality: (+)-camphorAbsolute configuration: (1S,2R,3S,4R)
(1S,2R,3S,4R)-3-Butoxy-N-(2-(diphenylphosphino)benzyl)-N-ethyl-4,7,7-trimethylbicyclo[2.2.1]heptan-2-amineC35H46NOPDe >99% (NMR)[α]D20=+46.1 (c 0.31, CHCl3)Source of chirality: (+)-camphorAbsolute configuration: (1S,2R,3S,4R)
(1S,2R,3S,4R)-3-(Benzyloxy)-N-(2-(diphenylphosphino)benzyl)-N-ethyl-4,7,7-trimethylbicyclo[2.2.1]heptan-2-amineC38H44NOPDe >99% (NMR)[α]D20=+63.8 (c 0.0.50, CHCl3)Source of chirality: (+)-camphorAbsolute configuration: (1S,2R,3S,4R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 20, 31 October 2013, Pages 1253–1256