| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343958 | 980052 | 2013 | 7 صفحه PDF | دانلود رایگان |
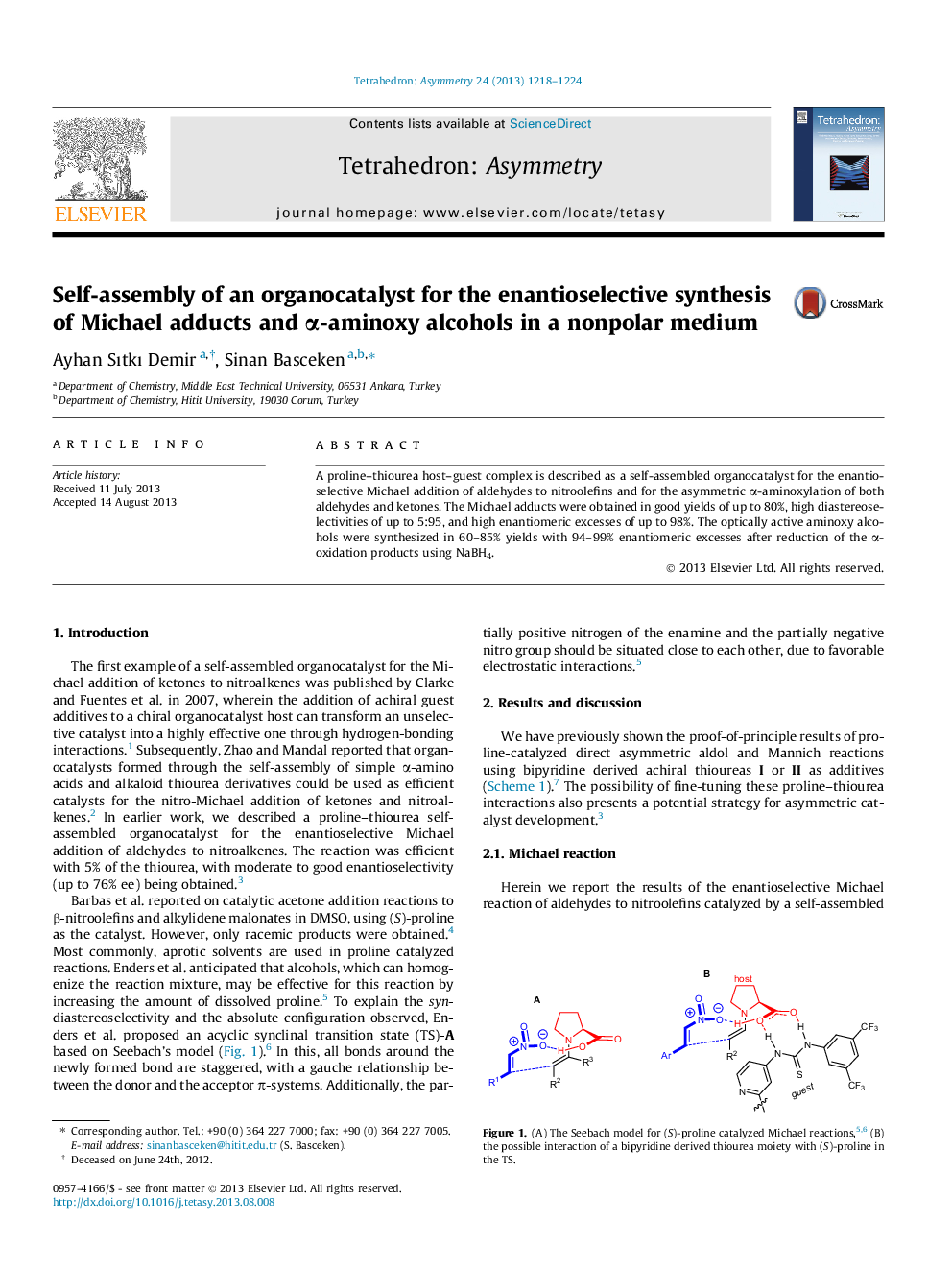
A proline–thiourea host–guest complex is described as a self-assembled organocatalyst for the enantioselective Michael addition of aldehydes to nitroolefins and for the asymmetric α-aminoxylation of both aldehydes and ketones. The Michael adducts were obtained in good yields of up to 80%, high diastereoselectivities of up to 5:95, and high enantiomeric excesses of up to 98%. The optically active aminoxy alcohols were synthesized in 60–85% yields with 94–99% enantiomeric excesses after reduction of the α-oxidation products using NaBH4.
Figure optionsDownload as PowerPoint slide
(2R,3S)-2-Methyl-4-nitro-3-phenylbutyraldehydeC11H13NO3ee = 93%[α]D25=+38.5 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-(4-Bromophenyl)-2-methyl-4-nitrobutyraldehydeC11H12BrNO3ee = 98%[α]D25=+36.7 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-3-(4-Methoxyphenyl)-2-methyl-4-nitrobutyraldehydeC12H15NO4ee = 90%[α]D25=+28.2 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-3-(2-Methoxyphenyl)-2-methyl-4-nitrobutyraldehydeC12H15NO4ee = 97%[α]D25=+24.2 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-(2-Bromophenyl)-2-methyl-4-nitrobutyraldehydeC11H12BrNO3[α]D25=+56.5 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-2-Ethyl-4-nitro-3-(4-bromophenyl)butyraldehydeC12H14BrNO3ee = 92%[α]D25=+36.8 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R,3S)-2-(Methylethyl)-4-nitro-3-(4-bromophenyl)butyraldehydeC13H16BrNO3ee = 95%[α]D25=+35.4 (c 1.50, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R,3S)
(2R)-2-AnilinoxypropanolC9H13NO2ee = 99%[α]D25=+29.0 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R)
(2R)-2-AnilinoxybutanolC10H15NO2ee = 98%[α]D25=+36.1 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R)
(2R)-3-Methyl-2-anilinoxybutanolC11H17NO2ee = 94%[α]D25=+33.6 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R)
(2R)-2-AnilinoxypentanolC11H17NO2ee = 98%[α]D25=+28.7 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R)
(2R)-2-AnilinoxycyclohexanoneC12H15NO2ee = 99%[α]D25=+122.1 (c 1.0, CHCl3)Source of Chirality: (S)-prolineAbsolute configuration: (2R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 19, 15 October 2013, Pages 1218–1224