| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1343960 | 980052 | 2013 | 7 صفحه PDF | دانلود رایگان |
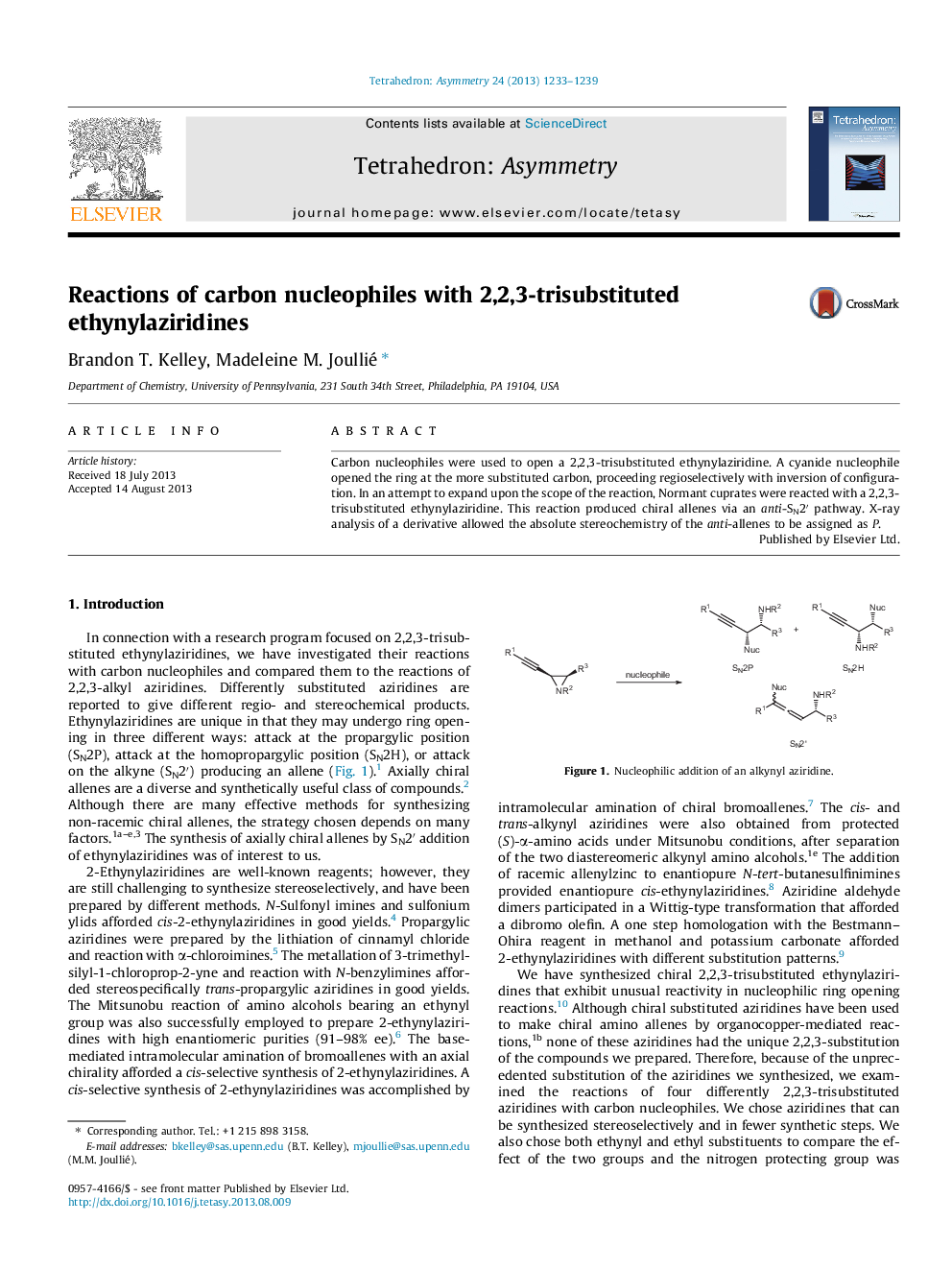
Carbon nucleophiles were used to open a 2,2,3-trisubstituted ethynylaziridine. A cyanide nucleophile opened the ring at the more substituted carbon, proceeding regioselectively with inversion of configuration. In an attempt to expand upon the scope of the reaction, Normant cuprates were reacted with a 2,2,3-trisubstituted ethynylaziridine. This reaction produced chiral allenes via an anti-SN2′ pathway. X-ray analysis of a derivative allowed the absolute stereochemistry of the anti-allenes to be assigned as P.
Figure optionsDownload as PowerPoint slide
N-((2R,3R)-1-((tert-Butyldimethylsilyl)oxy)-3-hydroxy-3-methylpentan-2-yl)-4-methylbenzenesulfonamideC19H35NO4SSi[α]D24=-30.7 (c 2.68, CHCl3)Absolute configuration: (2R,3R)Source of chirality: N-((2R,3R)-1,3-Dihydroxy-3-methylpentan-2-yl)-4-methylbenzenesulfonamide
N-((2R,3R)-1-((tert-Butyldimethylsilyl)oxy)-3-hydroxy-3-methylpent-4-yn-2-yl)-4-methylbenzenesulfonamideC19H31NO4SSi[α]D23=-23.4 (c 3.19, CHCl3)Absolute configuration: (2R,3R)Source of chirality: N-((2R,3R)-1,3-Dihydroxy-3-methylpent-4-yn-2-yl)-4-methylbenzenesulfonamide
(2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridineC19H29NO3SSi[α]D24=+22.5 (c 0.97, CHCl3)Absolute configuration: (2S,3S)Source of chirality: N-((2R,3R)-1-((tert-Butyldimethylsilyl)oxy)-3-hydroxy-3-methylpent-4-yn-2-yl)-4-methylbenzenesulfonamide
(2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethyl-2-methyl-1-tosylaziridineC19H33NO3SSi[α]D22=+14.7 (c 1.38, CHCl3)Absolute configuration: (2S,3S)Source of chirality: N-((2R,3R)-1-((tert-Butyldimethylsilyl)oxy)-3-hydroxy-3-methylpentan-2-yl)-4-methylbenzenesulfonamide
N-((2S,3R)-1-((tert-Butyldimethylsilyl)oxy)-3-cyano-3-methylpent-4-yn-2-yl)-2-nitrobenzenesulfonamideC19H27N3O5SSi[α]D19=-125.0 (c 1.60, CHCl3)Absolute configuration: (2S,3R)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-((2-nitrophenyl)sulfonyl)aziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylnona-3,4-dien-2-yl)-4-methylbenzenesulfonamideC23H39NO3SSi[α]D23=+63.9 (c 1.98, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methyl-5-phenylpenta-3,4-dien-2-yl)-4-methylbenzenesulfonamideC25H35NO3SSi[α]D23=+205 (c 3.30, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylhexa-3,4-dien-2-yl)-4-methylbenzenesulfonamideC20H33NO3SSi[α]D24=+59.4 (c 1.63, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3,6-dimethylhepta-3,4-dien-2-yl)-4-methylbenzenesulfonamideC22H37NO3SSi[α]D22=+76.7 (c 1.51, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-5-cyclopentyl-3-methylpenta-3,4-dien-2-yl)-4-methylbenzenesulfonamideC24H39NO3SSi[α]D22=+78.9 (c 1.95, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylhepta-3,4-dien-2-yl)-4-methylbenzenesulfonamideC21H35NO3SSi[α]D23=+65.8 (c 2.48, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-6-(3-Bromophenyl)-1-((tert-butyldimethylsilyl)oxy)-3-methylhexa-3,4-dien-2-yl)-4-methylbenzenesulfonamideC26H36BrNO3SSi[α]D20=+70.8 (c 1.68, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylocta-3,4,7-trien-2-yl)-4-methylbenzenesulfonamideC22H35NO3SSi[α]D22=+79.9 (c 0.90, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylhepta-3,4,6-trien-2-yl)-4-methylbenzenesulfonamideC21H33NO3SSi[α]D22=+57.0 (c 0.99, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
N-((2S,4S)-1-((tert-Butyldimethylsilyl)oxy)-3-methylhepta-3,4-dien-6-yn-2-yl)-4-methylbenzenesulfonamideC21H31NO3SSi[α]D24=+113.6 (c 1.38, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
(2S,4S)-3-Methyl-2-((4-methylphenyl)sulfonamido)-5-phenylpenta-3,4-dien-1-yl 3,5-dinitrobenzoateC26H23N3O8S[α]D23=+185.6 (c 1.09, CHCl3)Absolute configuration: (2S,4S)Source of chirality: (2S,3S)-3-(((tert-Butyldimethylsilyl)oxy)methyl)-2-ethynyl-2-methyl-1-tosylaziridine
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 19, 15 October 2013, Pages 1233–1239