| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344037 | 1500357 | 2012 | 7 صفحه PDF | دانلود رایگان |
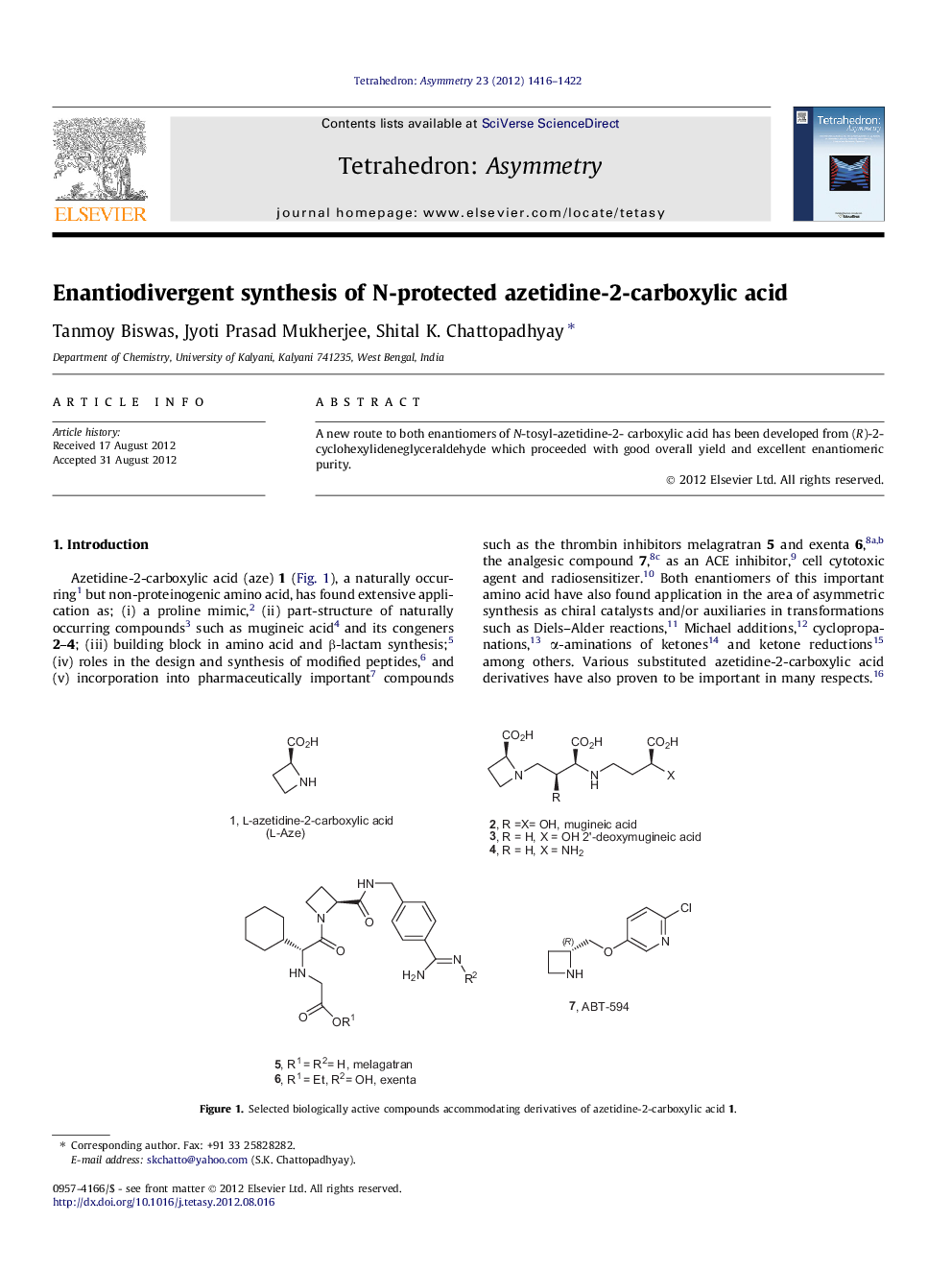
A new route to both enantiomers of N-tosyl-azetidine-2- carboxylic acid has been developed from (R)-2-cyclohexylideneglyceraldehyde which proceeded with good overall yield and excellent enantiomeric purity.
Figure optionsDownload as PowerPoint slide
(R)-N-Benzyl-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)but-3-en-1-amineC19H27NO2Source of chirality: d-mannitol[α]D = −12.0 (c 2.4, CHCl3)Absolute configuration: (1R,2′S)
Benzyl(R)-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)but-3-enyl(benzyl)carbamateC27H33NO4Source of chirality: d-mannitol[α]D25=+4.3 (c 1.00, CHCl3)Absolute configuration: (1R,2′S)
Benzyl benzyl((R)-3-hydroxy-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)propyl)carbamateC26H33NO5Source of chirality: d-mannitol[α]D = +20.0 (c 1.00, CHCl3)Absolute configuration: (1R,2′S)
(R)-3-Amino-3-((S)-1,4-dioxaspiro[4.5]decan-2-yl)propan-1-olC11H21NO3Source of chirality: d-mannitol[α]D = −6.95 (c 0.75, CHCl3)Absolute configuration: (3R,1′S)
(R)-2-((S)-1,4-Dioxaspiro[4.5]decan-2-yl)-1-tosylazetidineC18H25NO4SSource of chirality: d-mannitol[α]D = +91.5 (c 0.79, CH3OH).Absolute configuration: (2R,1′S)
(S)-1-((R)-1-Tosylazetidin-2-yl)ethane-1,2-diolC12H17NO4SSource of chirality: d-mannitol[α]D = +102.5 (c 0.86, CH3OH)Absolute configuration: (2R,1′S)
(S)-N-Benzyl-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)but-3-en-1-amineC19H27NO2Source of chirality: d-mannitol[α]D = +18.0 (c 2.7, CHCl3)Absolute configuration: (1S,2′S)
Benzyl (S)-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)but-3-enyl(benzyl)carbamateC27H33NO4Source of chirality: d-mannitol[α]D = +22.95 (c 1.0, CHCl3)Absolute configuration: (1S,2′S)
Benzyl benzyl((S)-3-hydroxy-1-((S)-1,4-dioxaspiro[4.5]decan-2-yl)propyl)carbamateC26H33NO5Source of chirality: d-mannitol[α]D = +2.9 (c 0.80, CHCl3)Absolute configuration: (1S,2′S)
(S)-3-Amino-3-((S)-1,4-dioxaspiro[4.5]decan-2-yl)propan-1-olC11H21NO3Source of chirality: d-mannitol[α]D = +6.4 (c 0.78, CHCl3)Absolute configuration: (3S,1′S)
(S)-2-((S)-1,4-Dioxaspiro[4.5]decan-2-yl)-1-tosylazetidineC18H25NO4SSource of chirality: d-mannitol[α]D = −77.0 (c 0.82, CHCl3)Absolute configuration: (2S,1′S)
(S)-1-((S)-1-Tosylazetidin-2-yl) ethane-1,2-diolC12H17NO4SSource of chirality: d-mannitol[α]D = −45.6 (c 0.60, CHCl3)Absolute configuration: (2S,1′S)
(S)-1-Tosylazetidine-2-carboxylic acidC11H13NO4SSource of chirality: d-mannitol[α]D = −149.2 (c 0.62, CHCl3)Absolute configuration: (1S)
(S)-Methyl 1-tosylazetidine-2-carboxylateC12H15NO4SSource of chirality: d-mannitol[α]D = −146.5 (c 0.51, CHCl3)Absolute configuration: (1S)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 18–19, 15 October 2012, Pages 1416–1422