| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344201 | 980078 | 2012 | 5 صفحه PDF | دانلود رایگان |
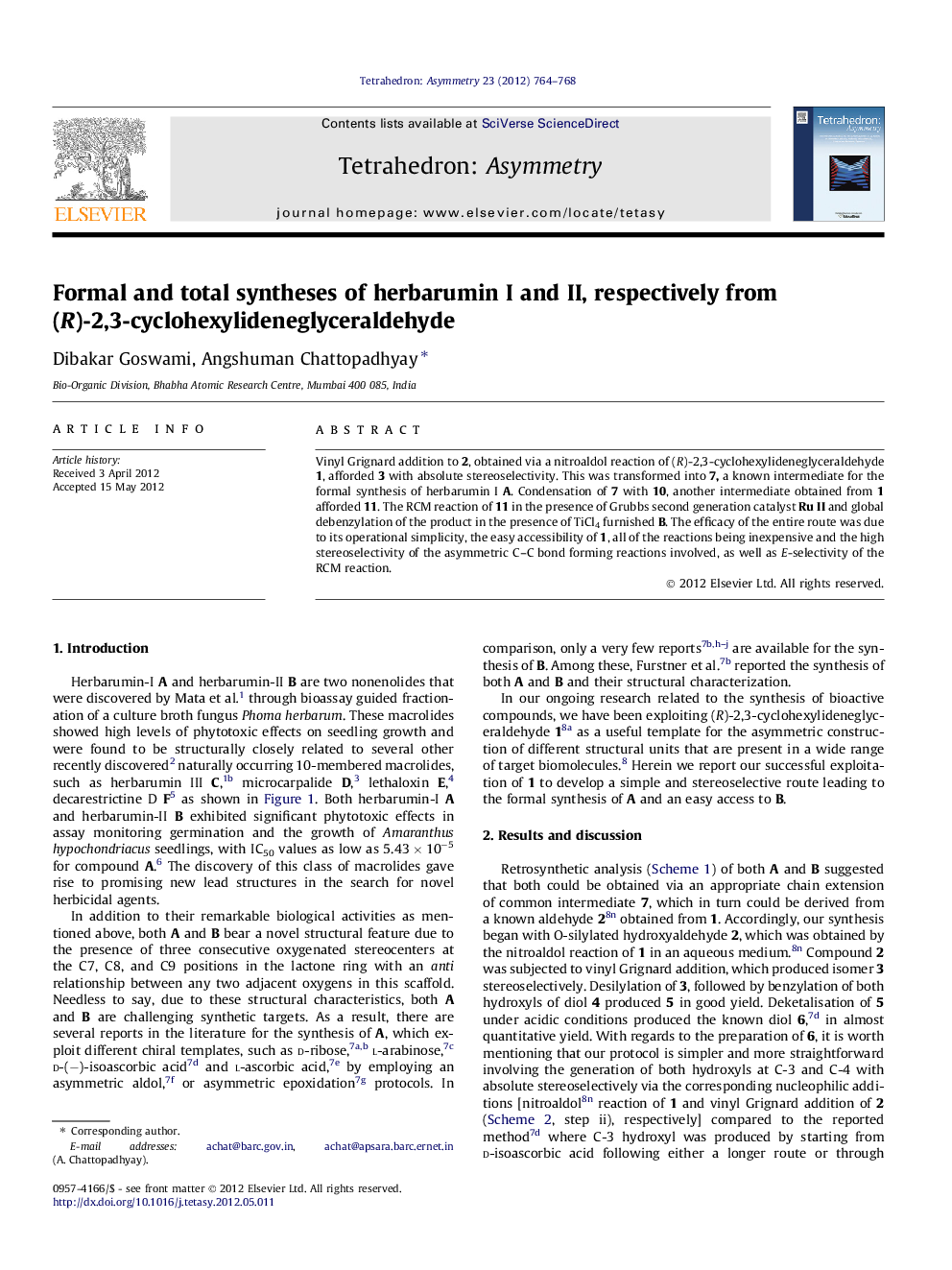
Vinyl Grignard addition to 2, obtained via a nitroaldol reaction of (R)-2,3-cyclohexylideneglyceraldehyde 1, afforded 3 with absolute stereoselectivity. This was transformed into 7, a known intermediate for the formal synthesis of herbarumin I A. Condensation of 7 with 10, another intermediate obtained from 1 afforded 11. The RCM reaction of 11 in the presence of Grubbs second generation catalyst Ru II and global debenzylation of the product in the presence of TiCl4 furnished B. The efficacy of the entire route was due to its operational simplicity, the easy accessibility of 1, all of the reactions being inexpensive and the high stereoselectivity of the asymmetric C–C bond forming reactions involved, as well as E-selectivity of the RCM reaction.
Figure optionsDownload as PowerPoint slide
C18H34O4Si(2R,3S,4S)-1,2-Cyclohexylidene-3-O-tert-butyldimethylsilylhex-5-ene-1,2,3,4-tetrol[α]D24=+11.3 (c 1.2, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3S,4S)
C12H20O4(2R,3S,4S)-1,2-Cyclohexylidenehex-5-ene-1,2,3,4-tetrol[α]D22=+23.4 (c 1.4, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3S,4S)
C26H32O4(2R,3S,4S)-1,2-Cyclohexylidene-3,4-O-dibenzylhex-5-ene-1,2,3,4-tetrol[α]D24 = +38.8 (c 1.06, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3S,4S)
C20H24O4(2R,3S,4S)-3,4-O-Dibenzylhex-5-ene-1,2,3,4-tetrol[α]D24 = +47.4 (c 1.6, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3S,4S)
C20H28O3(2R,3R)-1,2-Cyclohexylidene-3-O-benzylhept-6-ene-1,2,3-triol[α]D24=+13.4 (c 1.0, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3R)
C14H20O3(2R,3R)-3-O-Benzylhept-6-ene-1,2,3-triol[alpha]D24=+18.2 (c 1.2, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,3R)
C35H42O5(2R)-(4R,5R,6S)-5,6-Dibenzyloct-7-en-4-yl-2-benzylhex-5-enoate[α]D22=+68.4 (c 1.1, CHCl3)Source of chirality: (R)-2,3-cyclohexylideneglyceraldehydeAbsolute configuration: (2R,4R,5R,6S)
Journal: Tetrahedron: Asymmetry - Volume 23, Issue 10, 31 May 2012, Pages 764–768