| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344205 | 980078 | 2012 | 6 صفحه PDF | دانلود رایگان |
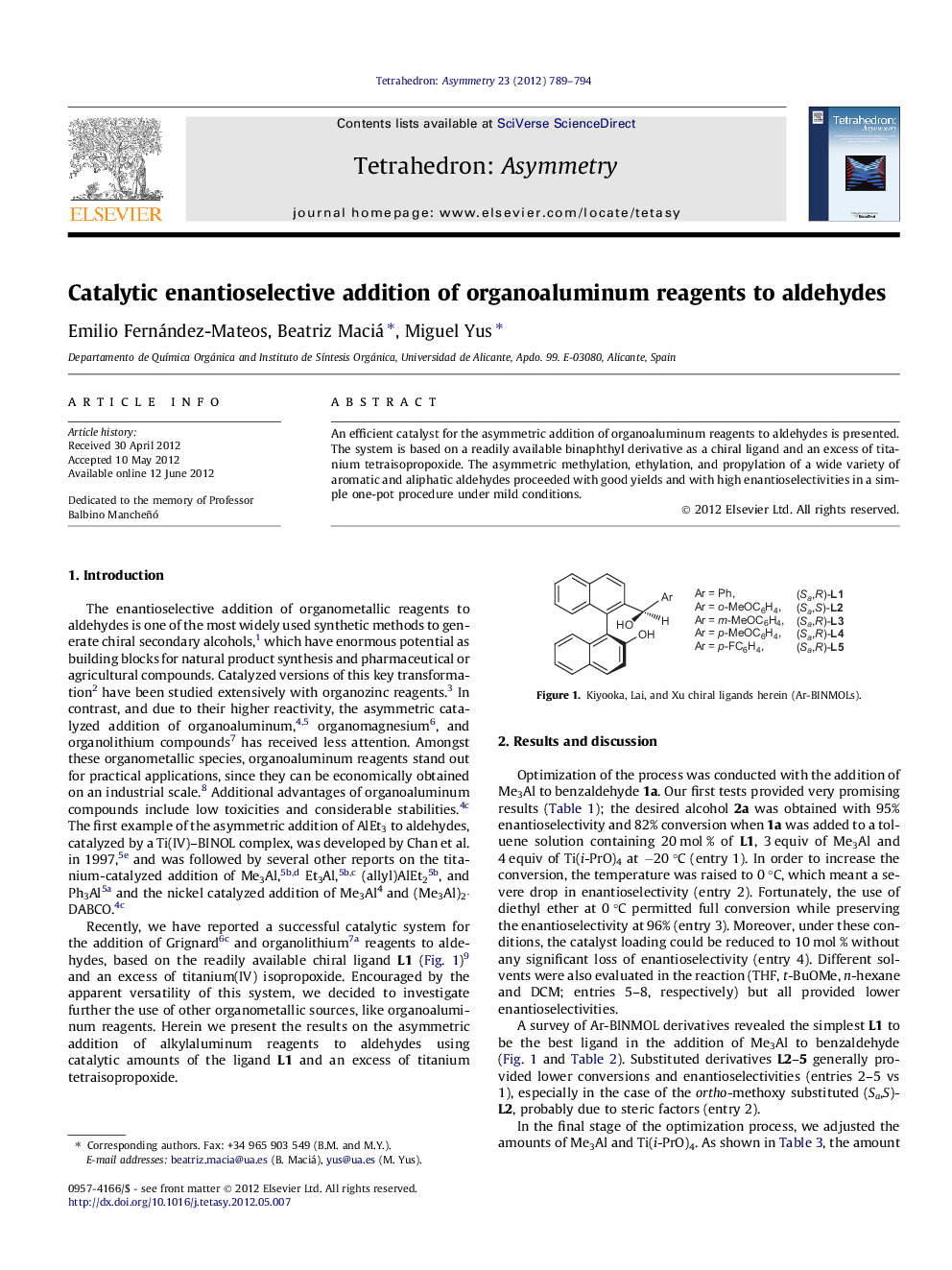
An efficient catalyst for the asymmetric addition of organoaluminum reagents to aldehydes is presented. The system is based on a readily available binaphthyl derivative as a chiral ligand and an excess of titanium tetraisopropoxide. The asymmetric methylation, ethylation, and propylation of a wide variety of aromatic and aliphatic aldehydes proceeded with good yields and with high enantioselectivities in a simple one-pot procedure under mild conditions.
Figure optionsDownload as PowerPoint slide
(S)-2′-(Benzyloxy)-(1,1′-binaphthalen)-2-olC27H20O2Ee = 100% (from HPLC)[α]D20=+5.2 (c 1.2, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S)
(Sa)-2′-((R)-Hydroxy(phenyl)methyl)-[1,1′-binaphthalen]-2-olC27H20O2Ee = 99% (from HPLC)[α]D20=+264.7 (c 1.0, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S,R)
(Sa)-2′-[(S)-Hydroxy(2-methoxyphenyl)methyl]-(1,1′-binaphthalen)-2-olC28H22O3Ee = 99% (from HPLC)[α]D20=+173.8 (c 1.0, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S,S)
(Sa)-2′-[(R)-Hydroxy(3-methoxyphenyl)methyl]-(1,1′-binaphthalen)-2-olC28H22O3Ee = 99% (from HPLC)[α]D20=+267.0 (c 1.0, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S,R)
(Sa)-2′-[(R)-Hydroxy(4-methoxyphenyl)methyl]-(1,1′-binaphthalen)-2-olC28H22O3Ee = 99% (from HPLC)[α]D20=+241.0 (c 0.5, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S,R)
(Sa)-2′-[(R)-(4-Fluorophenyl)(hydroxy)methyl]-(1,1′-binaphthalen)-2-olC27H19FO2Ee = 99% (from HPLC)[α]D20=+245.0 (c 1.0, CHCl3).Source of chirality: (Sa)-BINOLAbsolute configuration: (S,R)
(S)-1-PhenylethanolC8H10OEe = 94% (from GC)[α]D20=-57.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(o-Tolyl)ethanolC9H12OEe = 80% (from GC)[α]D20=-73.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(m-Tolyl)ethanolC9H12OEe = 94% (from GC)[α]D20=-51.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(p-Tolyl)ethanolC9H12O2Ee = 94% (from GC)[α]D20=-54.5 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-Methoxyphenyl)ethanolC9H12O2Ee = 94% (from GC)[α]D20=-44.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-(Trifluoromethyl)phenyl)ethanolC9H9F3OEe = 94% (from GC)[α]D20=-37.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-Chlorophenyl)ethanolC8H9ClOEe = 94% (from GC)[α]D20=-43.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-4-(1-Hydroxyethyl)benzonitrileC9H9NOEe = 94% (from GC)[α]D20=-49.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-[4-(1-Hydroxyethyl)phenyl]ethanoneC10H12O2Ee = 94% (from GC)[α]D20=-42.6 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(Naphthalen-2-yl)ethanolC12H12O2Ee = 94% (from GC)[α]D20=-46.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(Thiophen-2-yl)ethanolC6H8OSEe = 80% (from GC)[α]D20=-30.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(Furan-2-yl)ethanolC6H8O2Ee = 88% (from GC)[α]D20=-22.6 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S,E)-4-Phenylbut-3-en-2-olC10H12OEe = 90% (from GC)[α]D20=-29.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-4-Phenylbut-3-yn-2-olC10H10OEe = 62% (from HPLC)[α]D20=-28.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-Phenylpropan-2-olC9H12O2Ee = 84% (from GC)[α]D20=+44.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-3,3-Dimethylbutan-2-olC6H14OEe = 98% (from GC)[α]D20=-8.0 (c 1.7, EtOAc).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-Phenylpropan-1-olC9H12OEe = 90% (from GC)[α]D20=-38.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(p-Tolyl)propan-1-olC10H14OEe = 87% (from GC)[α]D20=-40.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-Chlorophenyl)propan-1-olC9H11ClOEe = 92% (from GC)[α]D20=-35.7 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-Chlorophenyl)butan-1-olC10H13ClOEe = 94% (from GC)[α]D20=-41.6 (c 1.3, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-(4-Methoxyphenyl)butan-1-olC11H16O2Ee = 92% (from GC)[α]D20=-35.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-1-Cyclohexylbutan-1-olC10H20OEe = 94% (from GC)[α]D20=-11.3 (c 0.9, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-Naphthalen-2-yl(phenyl)methanolC17H14OEe = 20% (from HPLC)[α]D20=+3.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
(S)-2,2-Dimethyl-1-phenylpropan-1-olC11H16OEe = 72% (from HPLC)[α]D20=-26.0 (c 1.0, CHCl3).Source of chirality: Ph-BINMOLAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 23, Issue 10, 31 May 2012, Pages 789–794