| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344370 | 1500363 | 2011 | 6 صفحه PDF | دانلود رایگان |
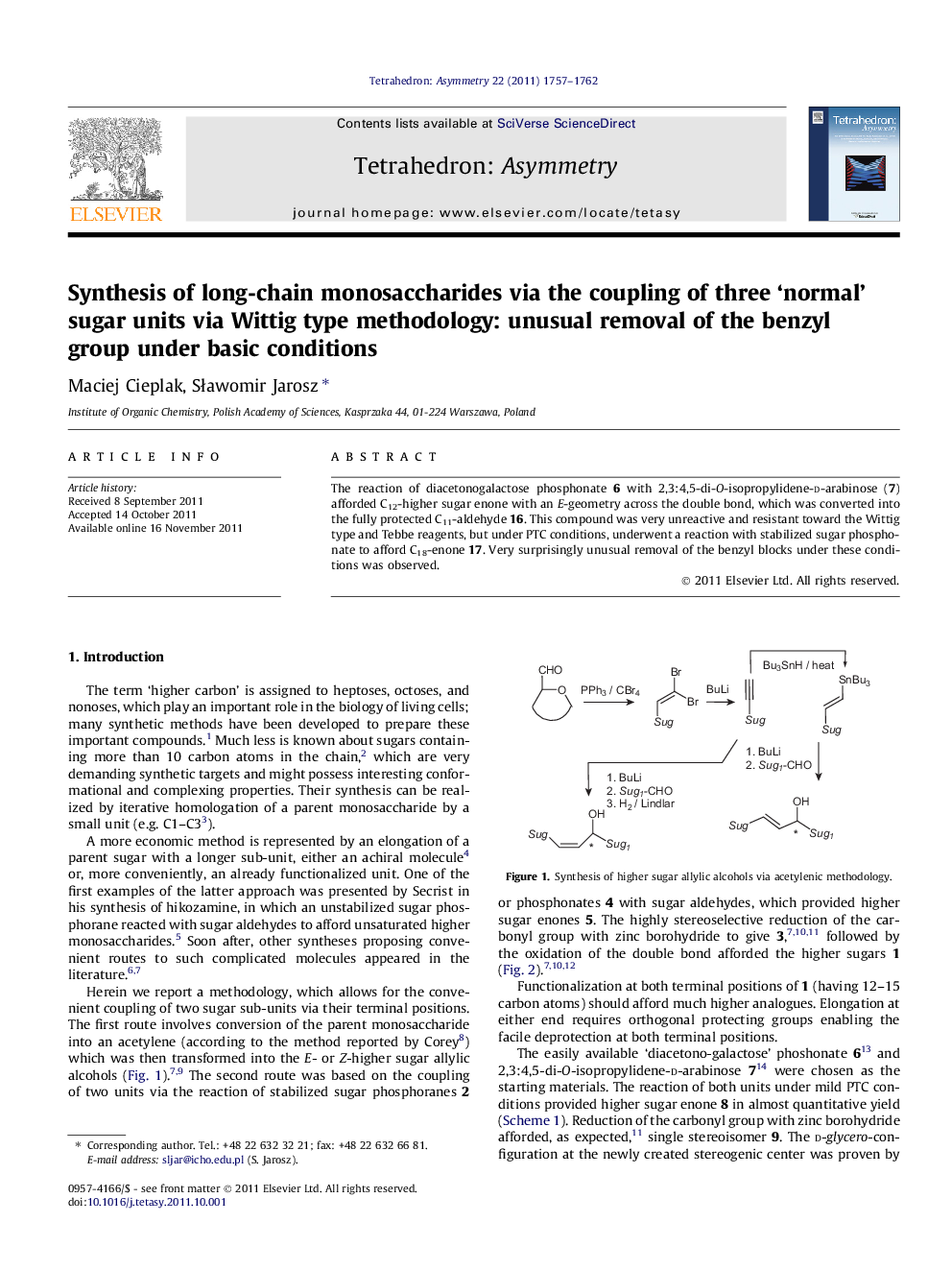
The reaction of diacetonogalactose phosphonate 6 with 2,3:4,5-di-O-isopropylidene-d-arabinose (7) afforded C12-higher sugar enone with an E-geometry across the double bond, which was converted into the fully protected C11-aldehyde 16. This compound was very unreactive and resistant toward the Wittig type and Tebbe reagents, but under PTC conditions, underwent a reaction with stabilized sugar phosphonate to afford C18-enone 17. Very surprisingly unusual removal of the benzyl blocks under these conditions was observed.
Figure optionsDownload as PowerPoint slide
1,2,3,4,9,10,11,12-Tetra-O-isopropylidene-7,8-dideoksy-7,8-didehydro-d-arabino-α-d-galacto-dodec-7(E)-ene-1,5-pyranose-6-uloseC24H36O10[α]D = −90.1 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5S,9R,10S,11R)
1,2,3,4,9,10,11,12-Tetra-O-isopropylidene-7,8-dideoxy-7,8-didehydro-d-gluko-α-d-galacto-dodec-7(E)-eno-1,5-pyranoseC24H38O10[α]D = −29.4 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,9R,10S,11R)
1,2,3,4,9,10,11,12-Tetra-O-isopropylidene-6-O-benzyl-7,8-dideoxy-7,8-didehydro-d-gluko-α-d-galacto-dodec-7(E)-ene-1,5-pyranoseC31H44O10[α]D = −23.8 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,9R,10S,11R)
1,2,3,4,9,10,11,12-Tetra-O-isopropylidene-6,7,8-tri-O-benzyl-d-manno-d-erytro-α-d-galacto-dodec-1,5-pyranoseC45H58O12[α]D = −26.3 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,7R,8R,9R,10R,11R)
1,2,3,4,9,10-Tri-O-isopropylidene-6,7,8-tri-O-benzyl-11,12-dihydroxy-d-manno-d-ertro-α-d-galacto-dodec-1,5-pyranoseC42H54O12[α]D = −55.9 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,7R,8R,9R,10R,11R)
1,2,3,4,9,10-Tri-O-isopropylidene-6,7,8-tri-O-benzyl-11-hydroxy-d-xylo-d-erytro-α-d-galacto-undec-1,5-pyranoseC41H52O11[α]D = −35 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,7R,8R,9R,10R)
1,2,3,4,9,10,15,16,17,18-penta-O-isopropylidene-6,7,8-tri-O-benzyl-11,12-dideoxy-11,12-didehydro-α-d-galacto-d-xylo-d-erytro-α-d-galacto-octadeka-1,5;14,18-dipyranose-13-uloseC54H68O16[α]D = −47.2 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,7R,8R,9R,10R,14S, 15S,16S,17R,18R)
1,2,3,4,9,10,15,16,17,18-penta-O-isopropylidene-6,8-di-O-benzyl-11,12-dideoxy-11,12-didehydro-α-d-galacto-d-xylo-d-glycero-α-d-galacto-octadeca-1,5:14,18-dipyranose-7,13-diuloseC47H60O16[α]D = −13.6 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6S,8S,9R,10R,14S,15S,16S,17R,18R)
1,2,3,4,9,10,15,16,17,18-penta-O-isopropylidene-6,7-di-O-benzyl-11,12-dideoxy-11,12-didehydro-α-d-galacto-d-threo-l-threo-α-d-galacto-octadeca-1,5;14,18-dipyranose-8,13-diuloseC47H60O16[α]D = −17.8 (c 1, CHCl3)Source of chirality: chiral poolAbsolute configuration: (1R,2R,3S,4S,5R,6R,7S,9S,10R,14S,15S,16S,17R,18R)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 18–19, 15 October 2011, Pages 1757–1762