| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344374 | 1500363 | 2011 | 6 صفحه PDF | دانلود رایگان |
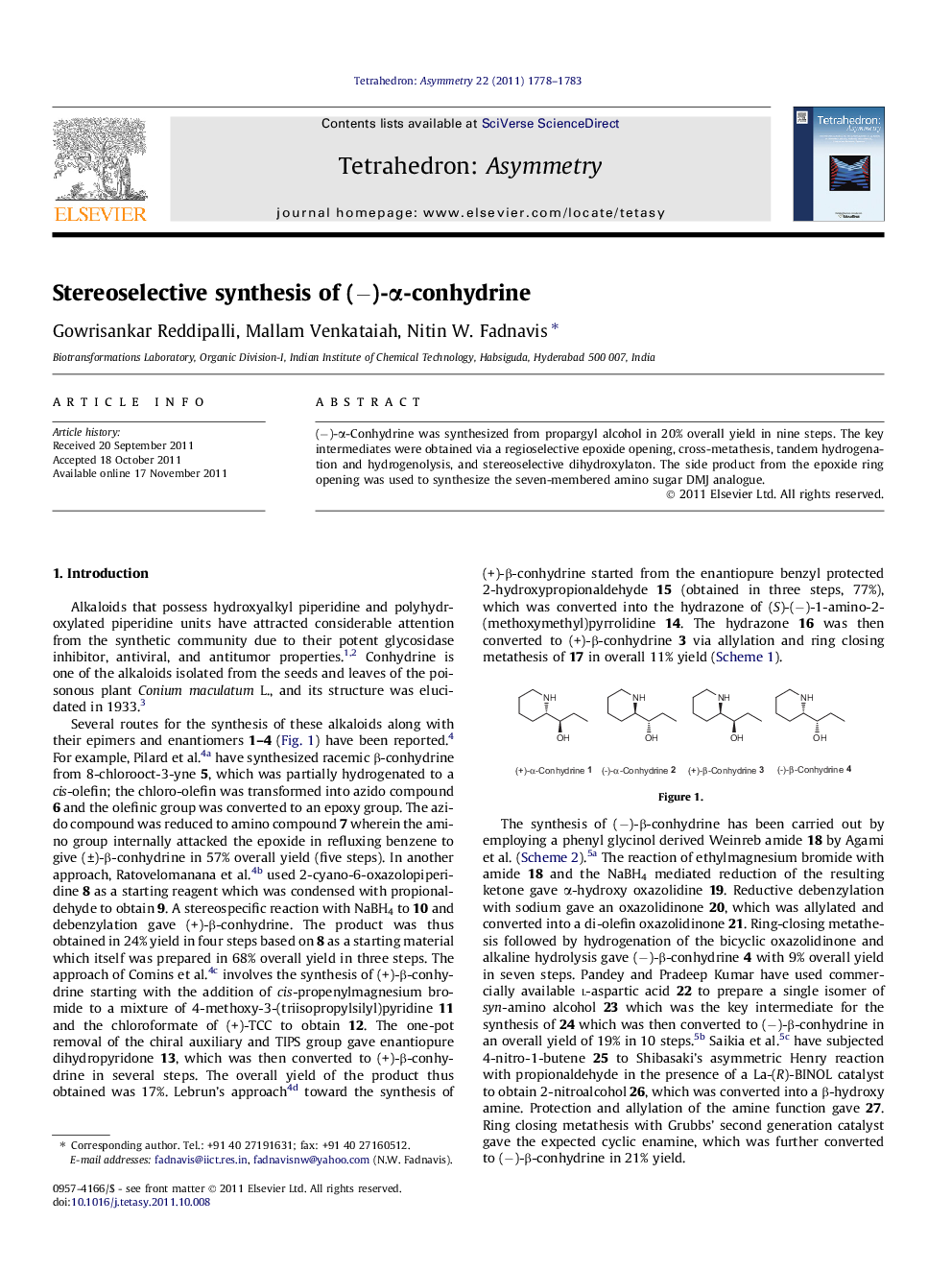
(−)-α-Conhydrine was synthesized from propargyl alcohol in 20% overall yield in nine steps. The key intermediates were obtained via a regioselective epoxide opening, cross-metathesis, tandem hydrogenation and hydrogenolysis, and stereoselective dihydroxylaton. The side product from the epoxide ring opening was used to synthesize the seven-membered amino sugar DMJ analogue.
Figure optionsDownload as PowerPoint slide
tert-Butyl allyl((1R)-1-((4R)-2-phenyl-1,3-dioxolan-4-yl)but-3-en-1-yl)carbamateC21H29NO4[α]D25=-32.3 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (1R,4R)
tert-Butyl allyl((4S,5R)-4-allyl-2-phenyl-1,3-dioxan-5-yl)carbamateC21H29NO4[α]D25=-29.3 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (4S,5R)
tert-Butyl allyl((2S,3S)-2-(benzyloxy)-1-hydroxyhex-5-en-3-yl)carbamateC21H31NO4[α]D25=-8.9 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,3S)
((2S,3S)-3-Allyloxiran-2-yl)methanolC6H10O2[α]D25=-34.6 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,3S)
(S)-tert-Butyl 6-((S)-1-(benzyloxy)-2-hydroxyethyl)-5,6-dihydropyridine-1(2H)-carboxylateC19H27NO4[α]D25=-3.3 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (1S,6S)
(S)-tert-Butyl 6-((R)-1-(benzyloxy)allyl)-5,6-dihydropyridine-1(2H)-carboxylateC20H27NO3[α]D25=+4.5 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (1R,6S)
(S)-1-((R)-Piperidin-2-yl)propan-1-olC8H17NO[α]D25=-9.1 (c 1, EtOH)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (1S,2R)
(4aR,9aS)-tert-Butyl 2-phenyl-4,4a,9,9atetrahydro-[1,3]dioxino[5,4-b]azepine-5(6H)-carboxylateC19H25NO4[α]D25=-72.0 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (4aR,9aS)
(4aR,7R,8S,9aS)-tert-Butyl 7,8-dihydroxy-2-phenylhexahydro-[1,3]dioxino[5,4-b]azepine-5(6H)-carboxylateC19H27NO6[α]D25=-42.1 (c 1, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (4aR,7R,8S,9aS)
(3R,4S,6S,7R)-7-(Hydroxymethyl)azepane-3,4,6-triolC7H15NO4[α]D25=-15.5 (c 1, MeOH)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (3R,4S,6S,7R)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 18–19, 15 October 2011, Pages 1778–1783