| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344728 | 1500369 | 2010 | 5 صفحه PDF | دانلود رایگان |
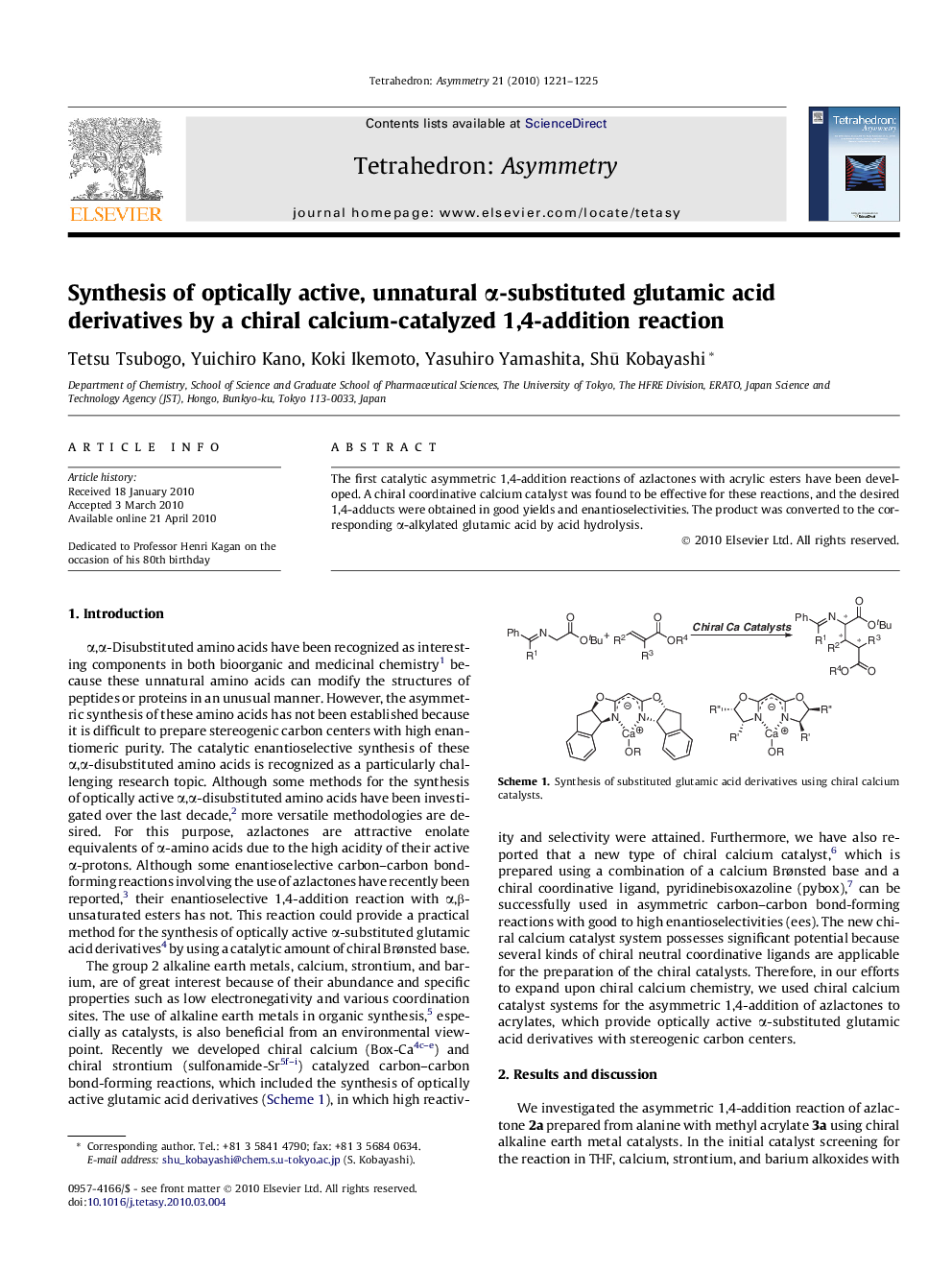
The first catalytic asymmetric 1,4-addition reactions of azlactones with acrylic esters have been developed. A chiral coordinative calcium catalyst was found to be effective for these reactions, and the desired 1,4-adducts were obtained in good yields and enantioselectivities. The product was converted to the corresponding α-alkylated glutamic acid by acid hydrolysis.
Figure optionsDownload as PowerPoint slide
(S)-Methyl 3-(4,5-dihydro-4-methyl-5-oxo-2-phenyloxazol-4-yl)propanoateC14H15NO4Ee = 80%[α]D25=+1.4 (c 0.98, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Methyl 3-(4,5-dihydro-4-ethyl-5-oxo-2-phenyloxazol-4-yl)propanoateC15H17NO4Ee = 70%[α]D25=-2.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Methyl 3-(4,5-dihydro-4-nor-propyl-5-oxo-2-phenyloxazol-4-yl)propanoateC16H19NO4Ee = 76%[α]D24=-2.7 (c 0.99, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Methyl 3-(4,5-dihydro-4-iso-butyl-5-oxo-2-phenyloxazol-4-yl)propanoateC17H21NO4Ee = 84%[α]D25=-11.4 (c 0.94, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Methyl 3-(4,5-dihydro-4-benzyl-5-oxo-2-phenyloxazol-4-yl)propanoateC20H19NO4Ee = 64%[α]D26=-41.0 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Ethyl 3-(4,5-dihydro-4-methyl-5-oxo-2-phenyloxazol-4-yl)propanoateC15H17NO4Ee = 81%[α]D28=-2.4 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-tert-Butyl 3-(4,5-dihydro-4-methyl-5-oxo-2-phenyloxazol-4-yl)propanoateC17H21NO4Ee = 69%[α]D28=-8.1 (c 0.94, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-Benzyl 3-(4,5-dihydro-4-methyl-5-oxo-2-phenyloxazol-4-yl)propanoateC20H19NO4Ee = 78%[α]D25=-3.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-2-Methylglutamic acidC6H11NO4[α]D20=-4.7 (c 0.19, H2O, 80% ee)[α]D15=+3.0 (c 2.0, 6 M HCl, 81% ee)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
2,6-Bis((S)-5,5-dimethyl-4-methyl-4,5-dihydrooxazol-2-yl)pyridineC17H23N3O2[α]D25=-38.6 (c 0.5, CHCl3)Source of chirality: (S)-alanineAbsolute configuration: (S,S)
2,6-Bis((S)-5,5-diethyl-4-methyl-4,5-dihydrooxazol-2-yl)pyridineC21H31N3O2[α]D25=-38.6 (c 0.5, CHCl3)Source of chirality: (S)-alanineAbsolute configuration: (S,S)
Journal: Tetrahedron: Asymmetry - Volume 21, Issues 9–10, 17 May 2010, Pages 1221–1225