| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344737 | 1500369 | 2010 | 4 صفحه PDF | دانلود رایگان |
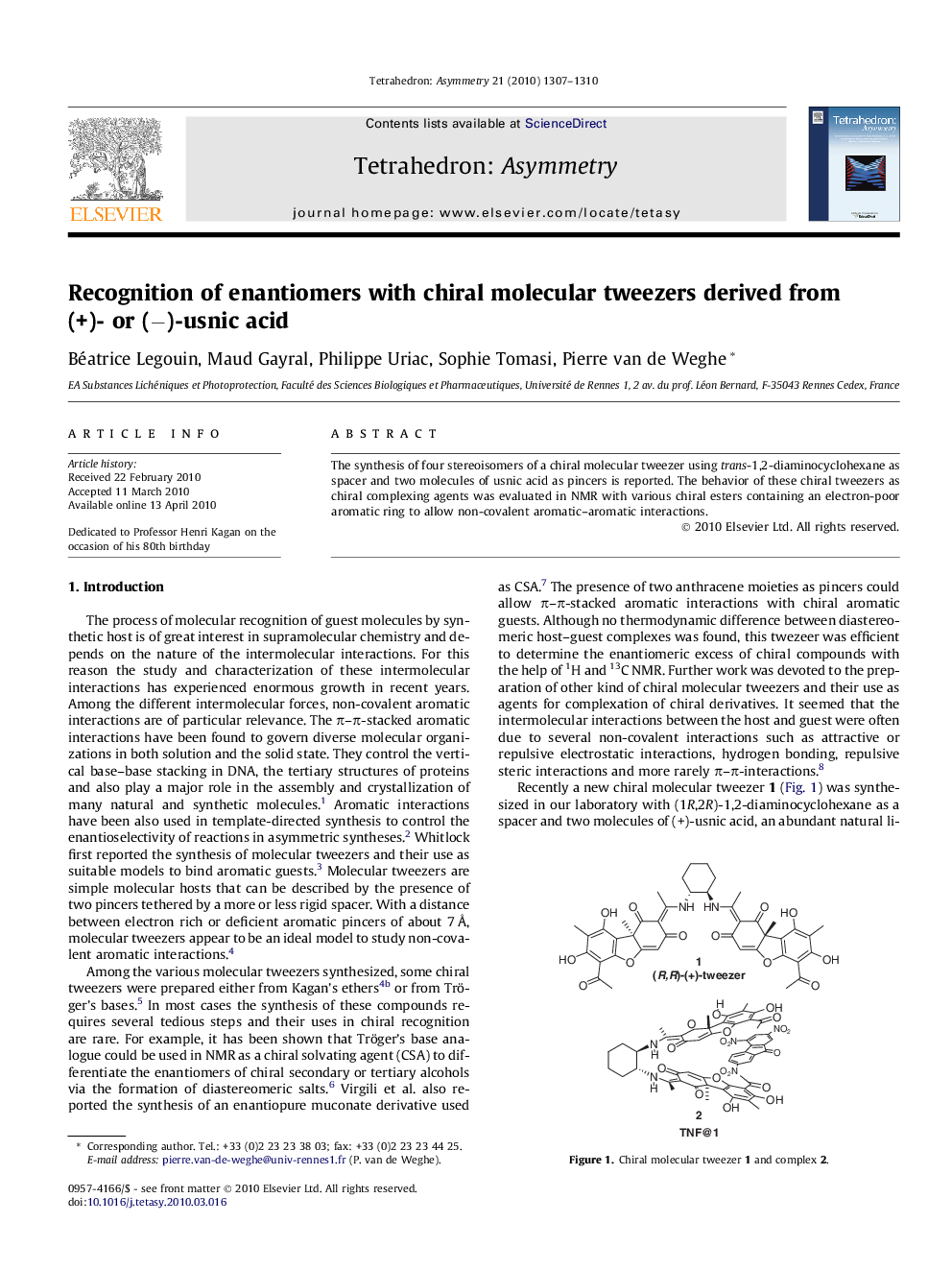
The synthesis of four stereoisomers of a chiral molecular tweezer using trans-1,2-diaminocyclohexane as spacer and two molecules of usnic acid as pincers is reported. The behavior of these chiral tweezers as chiral complexing agents was evaluated in NMR with various chiral esters containing an electron-poor aromatic ring to allow non-covalent aromatic–aromatic interactions.
Figure optionsDownload as PowerPoint slide
C42H42N2O12[α]D22=+234.5 (c 1.0, CH2Cl2)Source of chirality: (+)-usnic acid and (1R,2R)-1,2-diaminocyclohexaneAbsolute configuration: (R,R)
C42H42N2O12[α]D22=-443 (c 1.3, CH2Cl2)Source of chirality: (−)-usnic acid and (1R,2R)-1,2-diaminocyclohexaneAbsolute configuration: (R,R)
3,5-Dinitrobenzoic acid (1S)-1-phenylethyl esterC15H12N2O6[α]D22=+38.0 (c 1.0, CH2Cl2)Source of chirality: (S)-(−)-1-phenylethanolAbsolute configuration: (S)
(S)-sec-Butyl 3,5-dinitrobenzoateC11H12N2O6[α]D22=+29.0 (c 1.0, CH2Cl2)Source of chirality: (S)-(−)-2-butanolAbsolute configuration: (S)
C22H13N3O9[α]D22=-39.0 (c 1.0, CH2Cl2)Source of chirality: (S)-(+)-2-butanolAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 21, Issues 9–10, 17 May 2010, Pages 1307–1310