| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344750 | 980132 | 2010 | 6 صفحه PDF | دانلود رایگان |
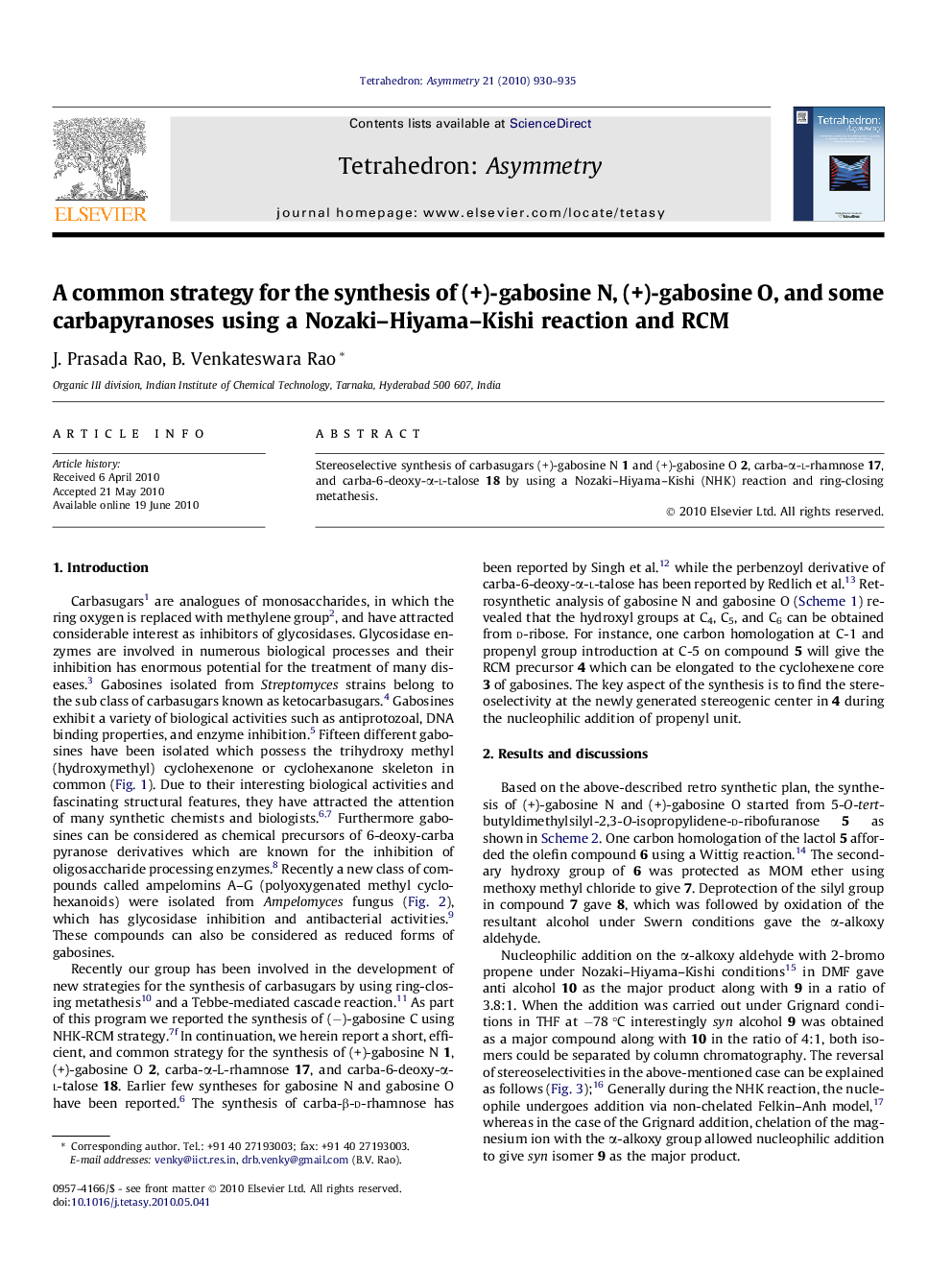
Stereoselective synthesis of carbasugars (+)-gabosine N 1 and (+)-gabosine O 2, carba-α-l-rhamnose 17, and carba-6-deoxy-α-l-talose 18 by using a Nozaki–Hiyama–Kishi (NHK) reaction and ring-closing metathesis.
Figure optionsDownload as PowerPoint slide
(R)-5-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-8,8,9,9-tetramethyl-2,4,7-trioxa-8-siladecaneC17H34O5Si[α]D28=-5.6 (c 0.92, CHCl3)Source of chirality: d-riboseAbsolute configuration: (5′R,4S,5S)
(R)-2-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-2-(methoxymethoxy)ethanolC11H20O5[α]D28=+76.1 (c 0.86, CHCl3)Source of chirality: d-riboseAbsolute configuration: (2R,4S,5S)
(1R,2S)-1-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-1-(methoxymethoxy)-3-methylbut-3-en-2-olC14H24O5[α]D28=-19.1 (c 1.52, CHCl3)Source of chirality: d-riboseAbsolute configuration: (1R,2S,4S,5S)
(1R,2R)-1-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-1-(methoxymethoxy)-3-methylbut-3-en-2-olC14H24O5[α]D28=+5.1 (c 1.4, CHCl3)Source of chirality: d-riboseAbsolute configuration: (1R,2R,4S,5S)
(3aS,4S,7aS)-4-(Methoxymethoxy)-2,2,6-trimethyl-3a,4-dihydrobenzo[d][1,3]dioxol-5(7aH)-oneC12H18O5[α]D28=-61.0 (c 0.8, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4S,7aS)
(3aS,4S,6R,7aS)-4-(Methoxymethoxy)-2,2,6-trimethyltetrahydrobenzo[d][1,3]dioxol-5(6H)-oneC12H20O5[α]D28=-36.6 (c 0.7, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4S,6R,7aS)
Gabosine NC7H10O4[α]D28=+172 (c 0.2, CH3OH)Source of chirality: d-riboseAbsolute configuration: (4S,5S,6S)
Gabosine OC7H12O4[α]D28=+17.5 (c 0.1, CH3OH)Source of chirality: d-riboseAbsolute configuration: (2S,3S,4S,6R)
(3aS,4R,5S,7aS)-4-(Methoxymethoxy)-2,2,6-trimethyl-3a,4,5,7a-tetrahydrobenzo[d][1,3]dioxol-5-olC12H20O5[α]D28=+27.6 (c 1.23, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4R,5S,7aS)
(3aS,4R,5R,7aS)-4-(Methoxymethoxy)-2,2,6-trimethyl-3a,4,5,7a-tetrahydrobenzo[d][1,3]dioxol-5-olC12H20O5[α]D28=-82.4 (c 1.2, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4R,5R,7aS)
(3aS,4R,5S,6R,7aS)-4-(Methoxymethoxy)-2,2,6-trimethylhexahydrobenzo[d][1,3]dioxol-5-olC12H22O5[α]D28=+3.0 (c 0.23, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4R,5S,6R,7aS)
(3aS,4R,5R,6R,7aS)-4-(Methoxymethoxy)-2,2,6-trimethylhexahydrobenzo[d][1,3]dioxol-5-olC12H22O5[α]D28=-39.8 (c 0.2, CHCl3)Source of chirality: d-riboseAbsolute configuration: (3aS,4R,5R,6R,7aS)
Carba-α-l-rhamnoseC7H14O4[α]D28=-5.4 (c 0.7, CH3OH)Source of chirality: d-riboseAbsolute configuration: (1S,2S,3R,4S,5R)
Carba-6-deoxy-α-l-taloseC7H14O4[α]D28=-2.58 (c 1.1, CH3OH)Source of chirality: d-riboseAbsolute configuration: (1S,2S,3R,4R,5R)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 8, 30 April 2010, Pages 930–935