| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344785 | 980137 | 2009 | 6 صفحه PDF | دانلود رایگان |
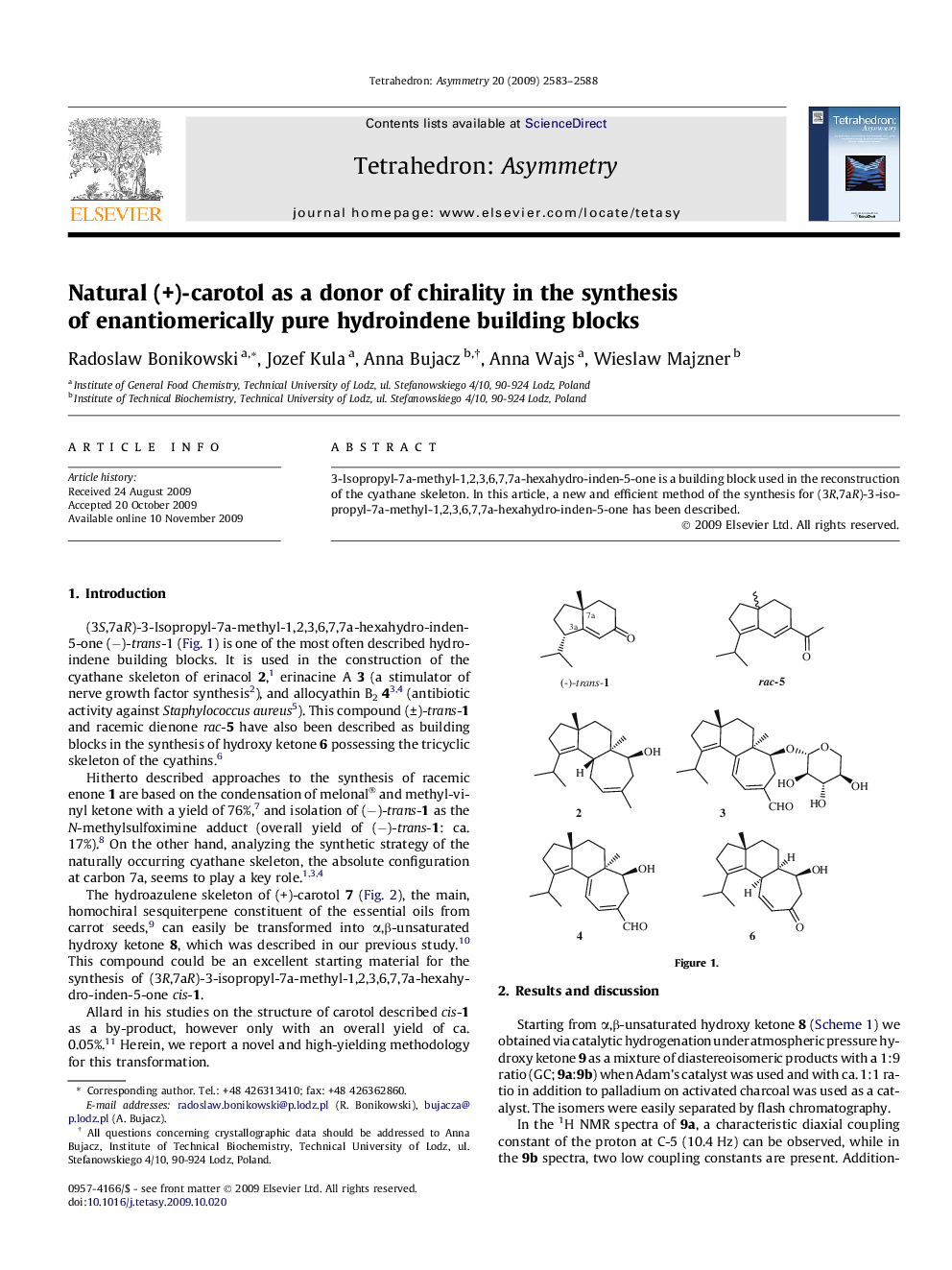
3-Isopropyl-7a-methyl-1,2,3,6,7,7a-hexahydro-inden-5-one is a building block used in the reconstruction of the cyathane skeleton. In this article, a new and efficient method of the synthesis for (3R,7aR)-3-isopropyl-7a-methyl-1,2,3,6,7,7a-hexahydro-inden-5-one has been described.
Figure optionsDownload as PowerPoint slide
1-((3R,3aS,5S,7aR)-3a-Hydroxy-3-isopropyl-7a-methyl-perhydroinden-5-yl)-ethanoneC15H26O2[α]D21=+7.6 (c 1.10, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5S,7aR)
1-((3R,3aS,5R,7aR)-3a-Hydroxy-3-isopropyl-7a-methyl-perhydroinden-5-yl)-ethanoneC15H26O2[α]D22=+5.8 (c 0.70, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5R,7aR)
(3R,3aS,5S,7aR)-3a-Hydroxy-3-isopropyl-7a-methyl-perhydroinden-5-yl acetateC15H26O3[α]D25=+23.6 (c 0.90, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5S,7aR)
(3R,3aS,5R,7aR)-3a-Hydroxy-3-isopropyl-7a-methyl-perhydroinden-5-yl acetateC15H26O3[α]D25=+24.1 (c 0.50, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5R,7aR)
(3R,3aS,5S,7aR)-3-Isopropyl-7a-methyl-perhydroinden-3a,5-diolC13H24O2[α]D24=+12.7 (c 1.20, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5S,7aR)
(3R,3aS,5R,7aR)-3-Isopropyl-7a-methyl-perhydroinden-3a,5-diolC13H24O2[α]D26=+14.3 (c 0.70, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,5R,7aR)
(3R,3aS,7aR)-3a-Hydroxy-3-isopropyl-7a-methyl-perhydroinden-5-oneC13H22O2[α]D23=+18.1 (c 0.90, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,3aS,7aR)
(3R,7aR)-3-Isopropyl-7a-methyl-1,2,3,6,7,7a-hexahydro-inden-5-oneC13H20O[α]D29=-38.2 (c 1.25, CHCl3)Source of chirality: (+)-carotolAbsolute configuration: (3R,7aR)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 22, 26 November 2009, Pages 2583–2588