| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344881 | 980153 | 2008 | 7 صفحه PDF | دانلود رایگان |
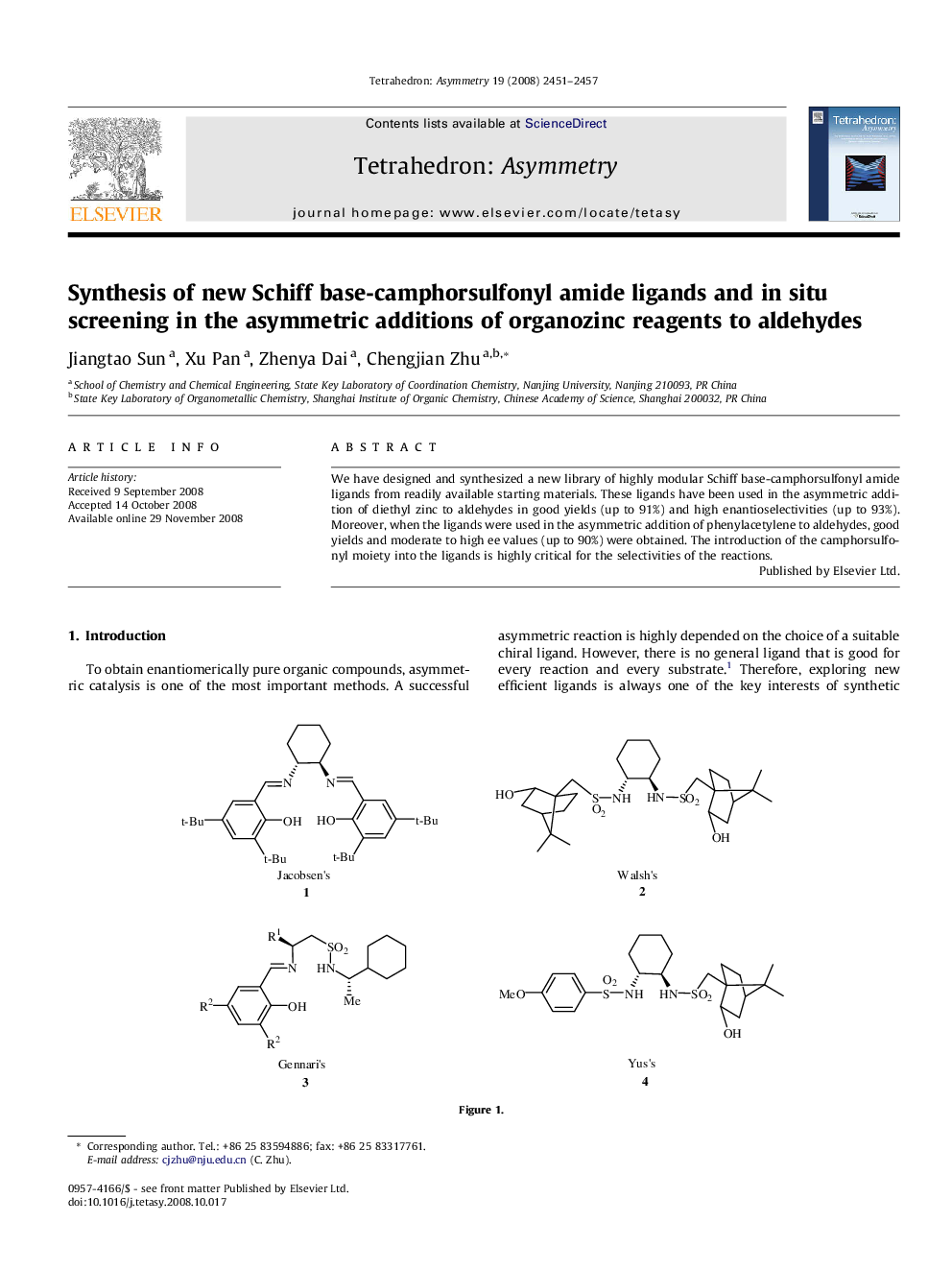
We have designed and synthesized a new library of highly modular Schiff base-camphorsulfonyl amide ligands from readily available starting materials. These ligands have been used in the asymmetric addition of diethyl zinc to aldehydes in good yields (up to 91%) and high enantioselectivities (up to 93%). Moreover, when the ligands were used in the asymmetric addition of phenylacetylene to aldehydes, good yields and moderate to high ee values (up to 90%) were obtained. The introduction of the camphorsulfonyl moiety into the ligands is highly critical for the selectivities of the reactions.
Figure optionsDownload as PowerPoint slide
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-2″-phenolC23H32N2O4S[α]D26=-59.0 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-methoxy-2″-phenolC24H34N2O5S[α]D26=-66.0 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″,5″-di-tert-butyl-2″-phenolC31H48N2O4S[α]D26=-44.8 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″,5″-dichloro-2″-phenolC23H30Cl2N2O4S[α]D26=-60.2 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-chloro-2″-phenolC23H31ClN2O4S[α]D26=-71.4 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-methyl-2″-phenolC24H34N2O4S[α]D26=-64.3 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-5″-tert-butyl-2″-phenolC27H40N2O4S[α]D26=-78.4 (c 0.2, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,4S,1′R,2′R)-N-{[trans-2′-(7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-naphthalen-2″-olC27H34N2O4S[α]D26=-28.5 (c 0.1, CH2Cl2)Absolute configuration: (1S,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-2″-phenolC23H34N2O4S[α]D26=-61.5 (c 0.1, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-methoxyl-2″-phenolC24H36N2O5S[α]D26=-42.2 (c 0.1, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″,5″-di-tert-butyl-2″-phenolC31H50N2O4S[α]D26=-49.0 (c 0.1, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″,5″-di-chloro-2″-phenolC23H32Cl2N2O4S[α]D26=-62.3 (c 0.2, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-chloro-2″-phenolC23H33ClN2O4S[α]D26=-45.9 (c 0.2, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-3″-methyl-2″-phenolC24H36N2O4S[α]D26=-64.3 (c 0.2, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-5″-tert-butyl-2″-phenolC27H42N2O4S[α]D26=-78.2 (c 0.2, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
(1S,2R,4S,1′R,2′R)-N-{[trans-2′-(2-Hydroxy-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl-methlsulfonamino)cyclohexylimino]methyl}-naphthalen-2″-phenolC27H36N2O4S[α]D26=-25.4 (c 0.1, CH2Cl2)Absolute configuration: (1S,2R,4S,1′R,2′R)Source of chirality: (R,R)-1,2-cyclohexyl-diamine; (1S)-(+)-10-camphorsulfonyl chloride
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 21, 3 November 2008, Pages 2451–2457