| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344883 | 980153 | 2008 | 8 صفحه PDF | دانلود رایگان |
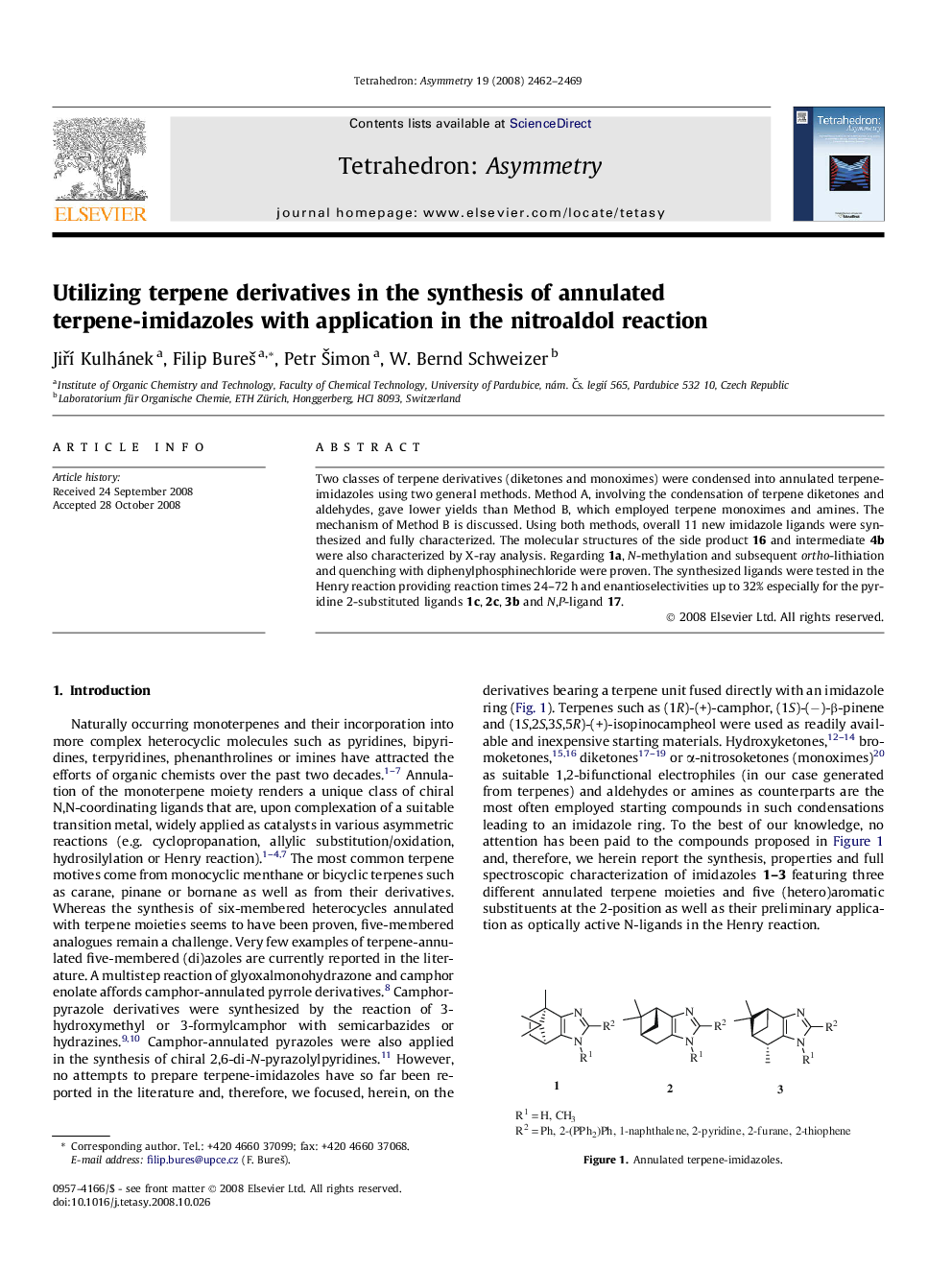
Two classes of terpene derivatives (diketones and monoximes) were condensed into annulated terpene-imidazoles using two general methods. Method A, involving the condensation of terpene diketones and aldehydes, gave lower yields than Method B, which employed terpene monoximes and amines. The mechanism of Method B is discussed. Using both methods, overall 11 new imidazole ligands were synthesized and fully characterized. The molecular structures of the side product 16 and intermediate 4b were also characterized by X-ray analysis. Regarding 1a, N-methylation and subsequent ortho-lithiation and quenching with diphenylphosphinechloride were proven. The synthesized ligands were tested in the Henry reaction providing reaction times 24–72 h and enantioselectivities up to 32% especially for the pyridine 2-substituted ligands 1c, 2c, 3b and N,P-ligand 17.
Figure optionsDownload as PowerPoint slide
(1R,7S)-1,10,10-Trimethyl-4-phenyl-3,5-diazatricyclo[5.2.1.02.6]deca-2(6),3-dieneC17H20N2[α]D20=+46.3 (c 1.0, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1R,7S)-1,10,10-Trimethyl-4-naphthalen-2-yl-3,5-diazatricyclo[5.2.1.02.6]deca-2(6),3-dieneC21H22N2[α]D20=+41.4 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1R,7S)-1,10,10-Trimethyl-4-pyridin-2-yl-3,5-diazatricyclo[5.2.1.02.6]deca-2(6),3-dieneC16H19N3[α]D20=+56.0 (c 1.0, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1R,7S)-1,10,10-Trimethyl-4-thiophen-2-yl-3,5-diazatricyclo[5.2.1.02.6]deca-2(6),3-dieneC15H18N2S[α]D20=+53.0 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1R,8R)-9,9-Dimethyl-4-phenyl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC16H18N2[α]D20=-26.2 (c 1.0, CH3OH)Source of chirality: (1S)-(−)-β-pineneAbsolute configuration: (1R,8R)
(1R,8R)-9,9-Dimethyl-4-naphthalen-1-yl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC20H20N2[α]D20=-21.1 (c 0.5, CH3OH)Source of chirality: (1S)-(−)-β-pineneAbsolute configuration: (1R,8R)
(1R,8R)-9,9-Dimethyl-4-pyridin-2-yl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC15H17N3[α]D20=-17.0 (c 1.0, CH3OH)Source of chirality: (1S)-(−)-β-pineneAbsolute configuration: (1R,8R)
(1R,8R)-9,9-Dimethyl-4-thiophen-2-yl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC14H16N2S[α]D20=-20.5 (c 0.5, CH3OH)Source of chirality: (1S)-(−)-β-pineneAbsolute configuration: (1R,8R)
(1R,8R)-4-Furan-2-yl-9,9-dimethyl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC14H16N2O[α]D20=+33.0 (c 0.5, CH3OH)Source of chirality: (1S)-(−)-β-pineneAbsolute configuration: (1R,8R)
(1S,7R,8S)-7,9,9-Trimethyl-4-phenyl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC17H20N2[α]D20=+55.0 (c 1.0, CH3OH)Source of chirality: (1S,2S,3S,5R)-(+)-isopinocampheolAbsolute configuration: (1S,7R,8S)
(1S,7R,8S)-7,9,9-Trimethyl-4-pyridin-2-yl-3,5-diazatricyclo[6.1.1.02.6]deca-2(6),3-dieneC16H19N3[α]D20=+8.0 (c 1.0, CH3OH)Source of chirality: (1S,2S,3S,5R)-(+)-isopinocampheolAbsolute configuration: (1S,7R,8S)
(1R,7S)-4-[2-(Diphenylphosphanyl)phenyl]-1,10,10-trimethyl-3,5-diazatricyclo[5.2.1.02.6]deca-2(6),3-dieneC30H31N2P[α]D20=+9.0 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1S,4R)-3-(Decylimino)-4,7,7-trimethylbicyclo[2.2.1]heptan-2-one oximeC20H36N2O[α]D20=+59.9 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1S,4R)-3-(Cyclohexylmethylimino)-4,7,7-trimethylbicyclo[2.2.1]heptan-2-one oximeC17H28N2O[α]D20=+72.4 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
(1S,4R)-3-(Furan-2-ylmethylimino)-4,7,7-trimethylbicyclo[2.2.1]heptan-2-one oximeC15H20N2O2[α]D20=+87.8 (c 0.5, CH3OH)Source of chirality: (1R)-(+)-camphorAbsolute configuration: (1R,7S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 21, 3 November 2008, Pages 2462–2469