| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344884 | 980153 | 2008 | 9 صفحه PDF | دانلود رایگان |
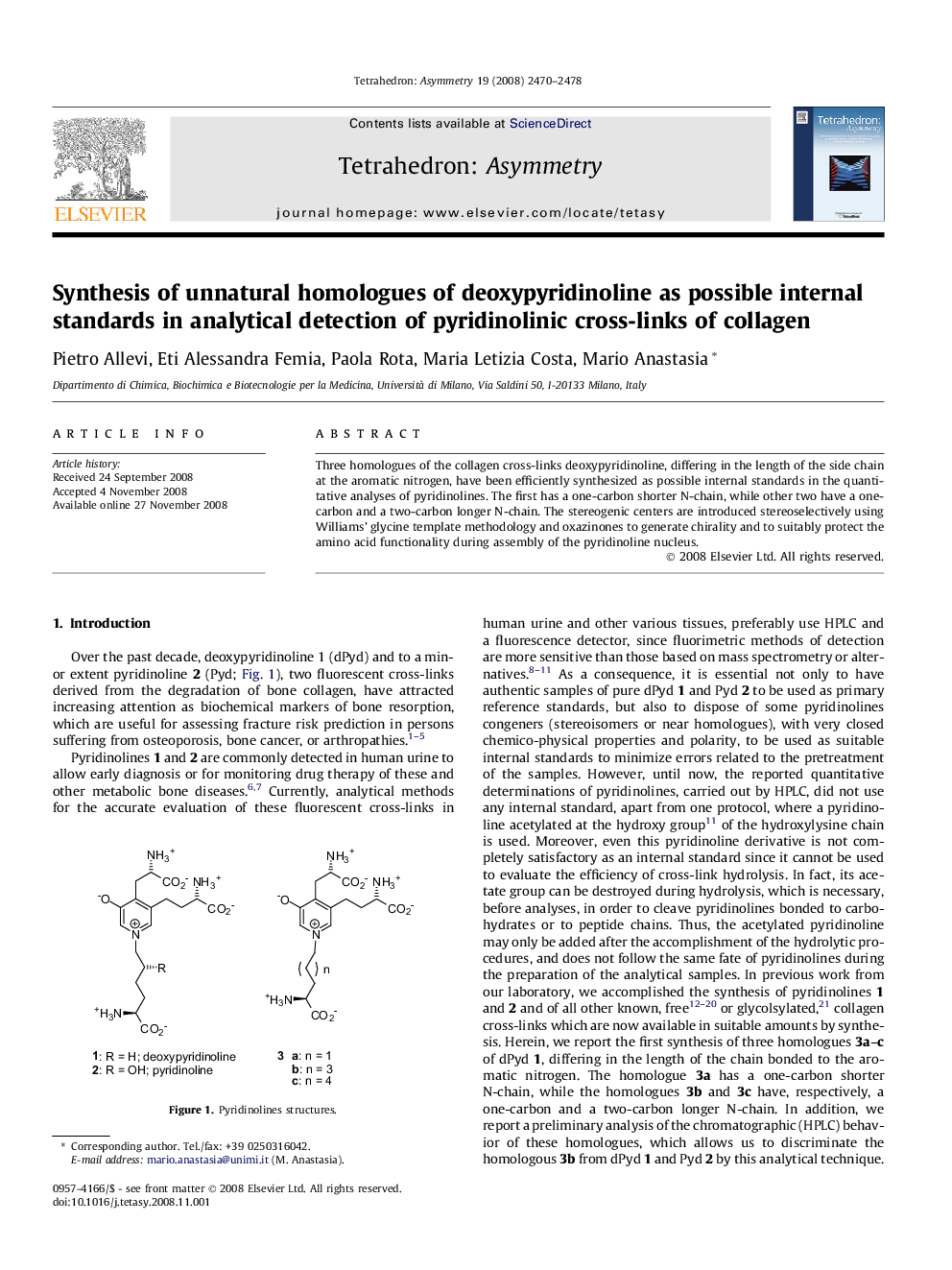
Three homologues of the collagen cross-links deoxypyridinoline, differing in the length of the side chain at the aromatic nitrogen, have been efficiently synthesized as possible internal standards in the quantitative analyses of pyridinolines. The first has a one-carbon shorter N-chain, while other two have a one-carbon and a two-carbon longer N-chain. The stereogenic centers are introduced stereoselectively using Williams’ glycine template methodology and oxazinones to generate chirality and to suitably protect the amino acid functionality during assembly of the pyridinoline nucleus.
Figure optionsDownload as PowerPoint slide
1-[(4S)-4-Benzyloxycarbonylamino-4-tert-butoxycarbonylbutyl]-4-[(2S)-2-benzyloxycarbonylamino-2-tert-butoxycarbonylethyl]-5-[(3S)-3-benzyloxycarbonylamino-3-tert-butoxycarbonylpropyl] pyridinium-3-olateC53H69N4O13[α]D20=+4.5 (c 1, CHCl3)Source of chirality: l-glutamic acidAbsolute configuration: (2S,3′S,4″S)
1-[(4S)-4-Amino-4-carboxylbutyl]-4-[(2S)-2-amino-2-carboxyethyl]-5-[(3S)-3-amino-3-carboxypropyl] pyridinium-3-olateC19H29F3N4O10[α]D20=+22.2 (c 0.9, H2O)Source of chirality: l-glutamic acidAbsolute configuration: (2S,3′S,4″S)
1-Benzyl-4-[(2S)-2-bis(tert-butoxycarbonylamino)-2-tert-butoxycarbonylethyl]-5-[(3S)-3-bis(tert-butoxycarbonylamino)-3-tert-butoxycarbonylpropyl] pyridinium-3-olateC47H71N3O13[α]D20=-34.0 (c 1, CHCl3)Source of chirality: l-glutamic acidAbsolute configuration: (2S,3′S)
4-[(2S)-2-Bis(tert-butoxycarbonylamino)-2-tert-butoxycarbonylethyl]-5-[(3S)-3-bis(tert-butoxycarbonylamino)-3-tert-butoxycarbonylpropyl]-3-hydroxypyridineC40H65N3O13[α]D20=-11.3 (c 1, CHCl3)Source of chirality: l-glutamic acidAbsolute configuration: (2S,3′S)
tert-Butyl (3S,5S,6R)-3-(5-azidopentyl)-2-oxo-5,6-diphenylmorpholine-4-carboxylateC26H32N4O4[α]D20=-61.2 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,5S,6R)
tert-Butyl (3S,5S,6R)-3-(5-aminopentyl)-2-oxo-5,6-diphenylmorpholine-4-carboxylateC26H34N2O4[α]D20=-54.9 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,5S,6R)
tert-Butyl (3S,5S,6R)-3-(6-iodohexyl)-2-oxo-5,6-diphenylmorpholine-4-carboxylateC27H34INO4[α]D20=-46.7 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,5S,6R)
1-[(3S,5S,6R)-5-(5,6-Dibenzyl-4-tert-butoxycarbonyl-2-oxomorpholin-3-yl)pentyl]-4-[(2S)-2-bis(tert-butoxycarbonylamino)-2-tert-butoxycarbonylethyl]-5-[(3S)-3-bis(tert-butoxycarbonylamino)-3-tert-butoxycarbonylpropyl] pyridinium-3-olateC66H96N4O17[α]D20=-26.7 (c 1, CHCl3)Source of chirality: l-glutamic acid, Williams glycine templateAbsolute configuration: (2S,3′S,3″S,5″S,6″R)
1-[(3S,5S,6R)-6-(5,6-Dibenzyl-4-tert-butoxycarbonyl-2-oxomorpholin-3-yl)hexyl]-4-[(2S)-2-bis(tert-butoxycarbonylamino)-2-tert-butoxycarbonylethyl]-5-[(3S)-3-bis(tert-butoxycarbonylamino)-3-tert-butoxycarbonylpropyl] pyridinium-3-olateC67H98N4O17[α]D20=-30.6 (c 1, CHCl3)Source of chirality: l-glutamic acid, Williams glycine templateAbsolute configuration: (2S,3′S,3″S,5″S,6″R)
1-[(3S,5S,6R)-5-(5,6-Dibenzyl-2-oxomorpholin-3-yl)pentyl]-4-[(2S)-2-amino-2-carboxyethyl]-5-[(3S)-3-amino-3-carboxypropyl] pyridinium-3-olateC41H44F12N4O15[α]D20=-10.6 (c 1, CD3OD)Source of chirality: l-glutamic acid, Williams glycine templateAbsolute configuration: (2S,3′S,3″S,5″S,6″R)
1-[(3S,5S,6R)-6-(5,6-Dibenzyl-2-oxomorpholin-3-yl)hexyl]-4-[(2S)-2-amino-2-carboxyethyl]-5-[(3S)-3-amino-3-carboxypropyl] pyridinium-3-olateC42H46F12N4O15[α]D20=-10.5 (c 1, CH3OH)Source of chirality: l-glutamic acid, Williams glycine templateAbsolute configuration: (2S,3′S,3″S,5″S,6″R)
1-[(6S)-6-Amino-6-carboxylhexyl]-4-[(2S)-2-amino-2-carboxyethyl]-5-[(3S)-3-amino-3-carboxypropyl] pyridinium-3-olateC19H30N4O7[α]D20=+15.2 (c 0.5, H2O)Source of chirality: l-glutamic acid, Williams glycine templateAbsolute configuration: (2S,3′S,6″S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 21, 3 November 2008, Pages 2470–2478