| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344926 | 980159 | 2008 | 6 صفحه PDF | دانلود رایگان |
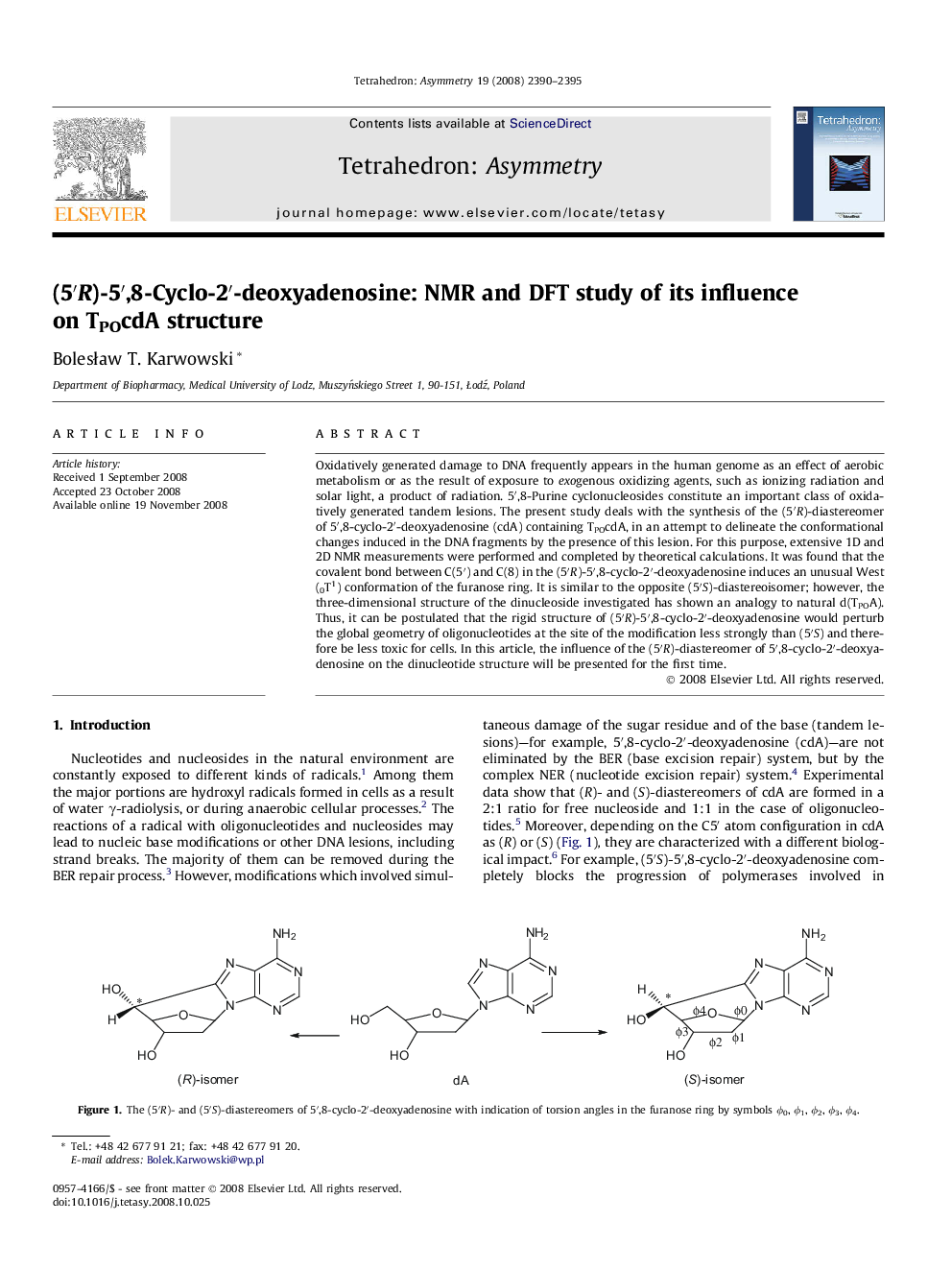
Oxidatively generated damage to DNA frequently appears in the human genome as an effect of aerobic metabolism or as the result of exposure to exogenous oxidizing agents, such as ionizing radiation and solar light, a product of radiation. 5′,8-Purine cyclonucleosides constitute an important class of oxidatively generated tandem lesions. The present study deals with the synthesis of the (5′R)-diastereomer of 5′,8-cyclo-2′-deoxyadenosine (cdA) containing TPOcdA, in an attempt to delineate the conformational changes induced in the DNA fragments by the presence of this lesion. For this purpose, extensive 1D and 2D NMR measurements were performed and completed by theoretical calculations. It was found that the covalent bond between C(5′) and C(8) in the (5′R)-5′,8-cyclo-2′-deoxyadenosine induces an unusual West (0T1) conformation of the furanose ring. It is similar to the opposite (5′S)-diastereoisomer; however, the three-dimensional structure of the dinucleoside investigated has shown an analogy to natural d(TPOA). Thus, it can be postulated that the rigid structure of (5′R)-5′,8-cyclo-2′-deoxyadenosine would perturb the global geometry of oligonucleotides at the site of the modification less strongly than (5′S) and therefore be less toxic for cells. In this article, the influence of the (5′R)-diastereomer of 5′,8-cyclo-2′-deoxyadenosine on the dinucleotide structure will be presented for the first time.
Graphical visualization of the difference in 3-D structure between TPO(5′S)cdA, d[TPOA], TPO(5′R)cdA.Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 20, 20 October 2008, Pages 2390–2395