| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344929 | 980159 | 2008 | 5 صفحه PDF | دانلود رایگان |
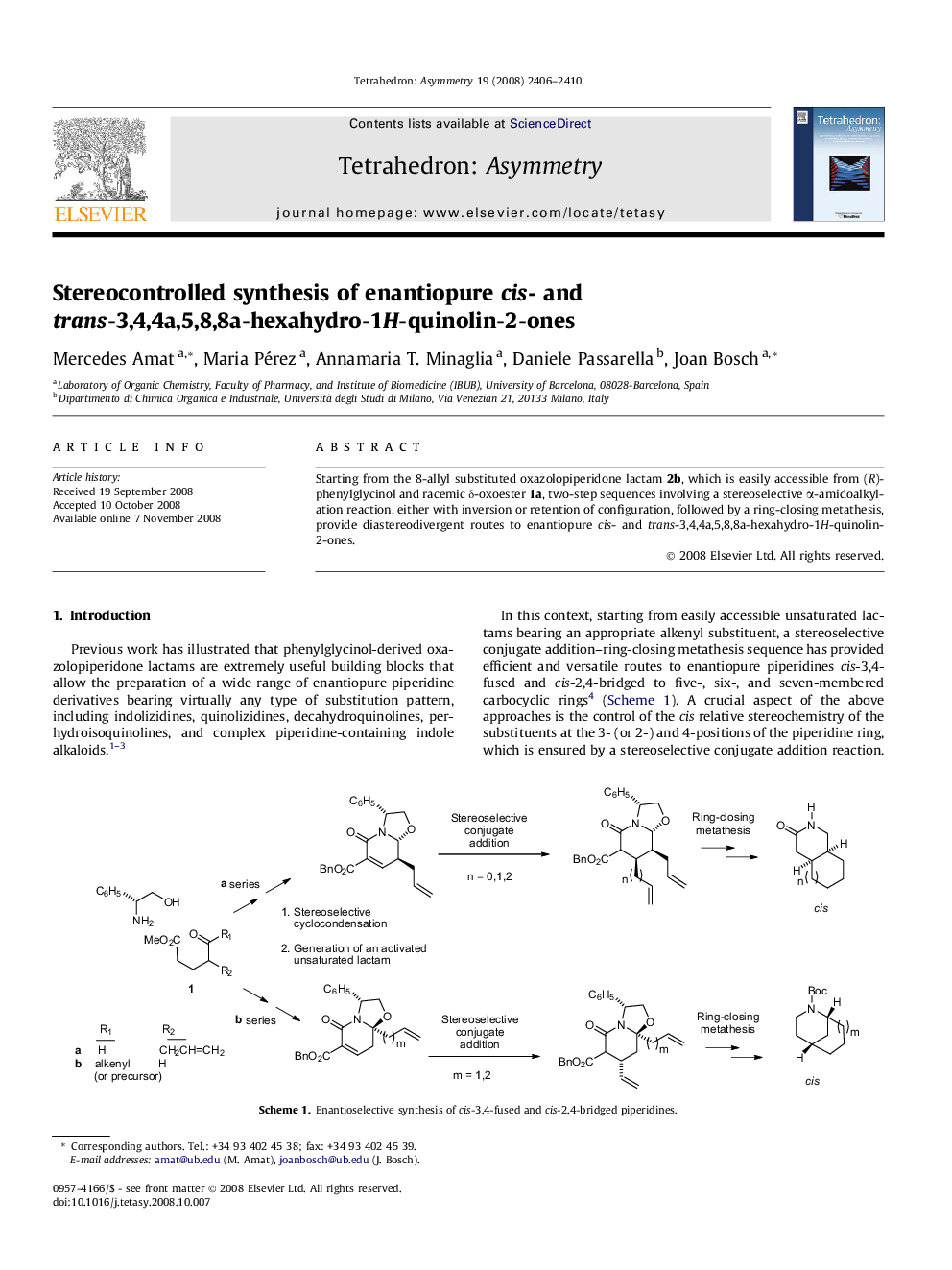
Starting from the 8-allyl substituted oxazolopiperidone lactam 2b, which is easily accessible from (R)-phenylglycinol and racemic δ-oxoester 1a, two-step sequences involving a stereoselective α-amidoalkylation reaction, either with inversion or retention of configuration, followed by a ring-closing metathesis, provide diastereodivergent routes to enantiopure cis- and trans-3,4,4a,5,8,8a-hexahydro-1H-quinolin-2-ones.
Figure optionsDownload as PowerPoint slide
(3R,8S,8aS)-8-Allyl-5-oxo-3-phenyl-2,3,6,7,8,8a-hexahydro-5H-oxazolo[3,2-a]pyridineC16H19NO2[α]D22=-59.9 (c 1.06, EtOH)Source of chirality: (R)-phenylglycinolAbsolute configuration: (3R,8S,8aS)
(3R,8R,8aS)-8-Allyl-5-oxo-3-phenyl-2,3,6,7,8,8a-hexahydro-5H-oxazolo[3,2-a]pyridineC16H19NO2[α]D22=-118.9 (c 1.0, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (3R,8R,8aS)
(5S,6S)-2,2,5,6-Tetraallyl-1-[(1R)-2-hydroxy-1-phenylethyl]piperidineC25H35NO[α]D22=+33.7 (c 1.5, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (5S,6S)
(4aS,8aR)-1-[(1R)-2-Hydroxy-1-phenylethyl]-3,4,4a,5,8,8a-hexahydro-1H-quinolin-2-oneC17H21NO2[α]D22=-39.6 (c 1.2, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (1R,4aS,8aR)
(4aS,8aS)1-[(1R)-2-Hydroxy-1-phenylethyl]-3,4,4a,5,8,8a-hexahydro-1H-quinolin-2-oneC17H21NO2[α]D22=+256.0 (c 0.8, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (1R,4aS,8aS)
(4aS,8aR)-3,4,4a,5,8,8a-Hexahydro-1H-quinolin-2-oneC9H13NO[α]D22=-33.9 (c 0.9, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (4aS,8aR)
(4aS,8aS)-3,4,4a,5,8,8a-Hexahydro-1H-quinolin-2-oneC9H13NO[α]D22=+186.5 (c 1.7, CHCl3)Source of chirality: (R)-phenylglycinolAbsolute configuration: (4aS,8aS)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 20, 20 October 2008, Pages 2406–2410