| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1344930 | 980159 | 2008 | 6 صفحه PDF | دانلود رایگان |
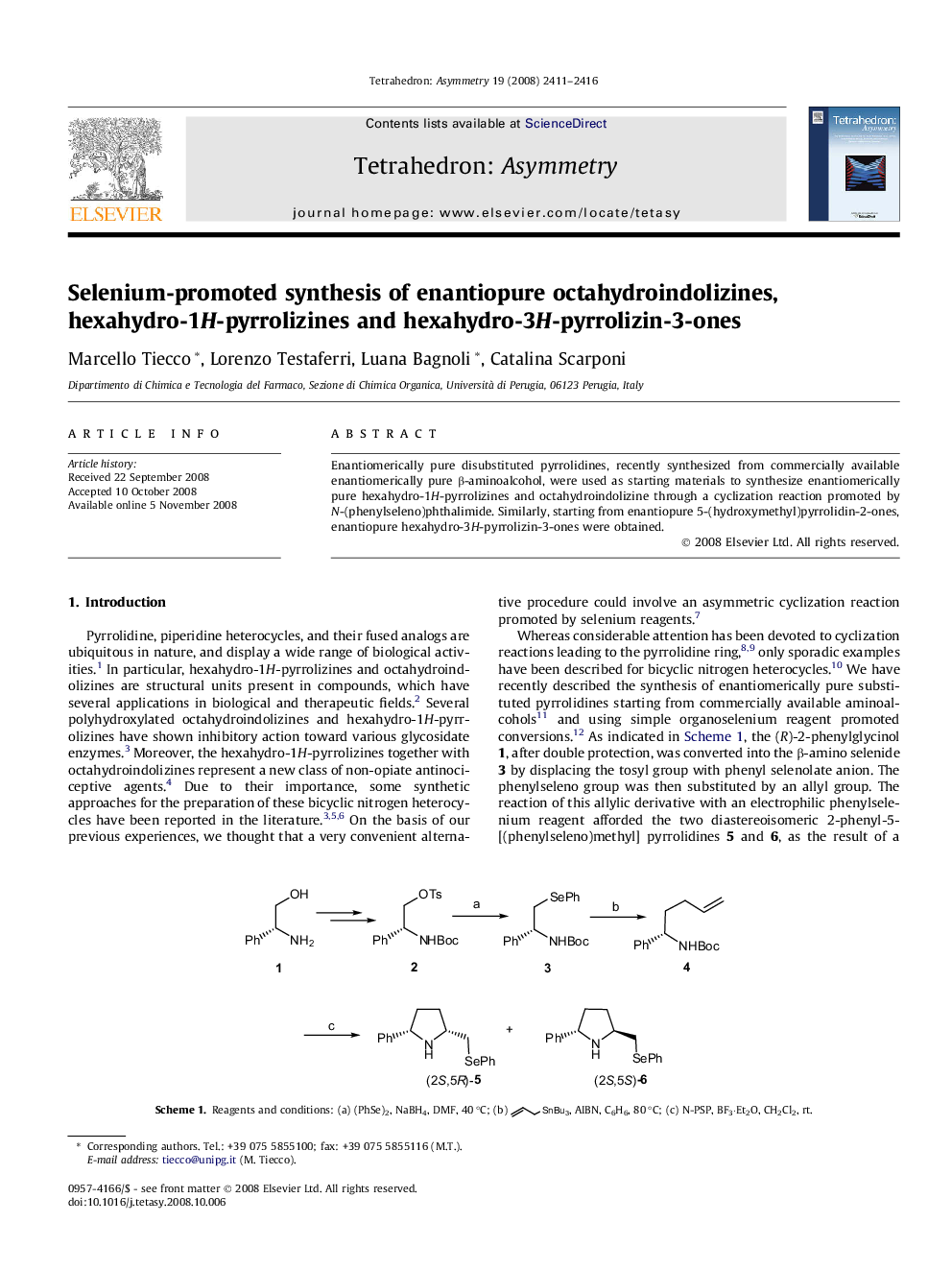
Enantiomerically pure disubstituted pyrrolidines, recently synthesized from commercially available enantiomerically pure β-aminoalcohol, were used as starting materials to synthesize enantiomerically pure hexahydro-1H-pyrrolizines and octahydroindolizine through a cyclization reaction promoted by N-(phenylseleno)phthalimide. Similarly, starting from enantiopure 5-(hydroxymethyl)pyrrolidin-2-ones, enantiopure hexahydro-3H-pyrrolizin-3-ones were obtained.
Figure optionsDownload as PowerPoint slide
(2S,5S)-2-But-3-en-1-yl-5-phenylpyrrolidineC14H19N[α]D30=-27.7 (c 2.09, CHCl3)Source of chirality: (R)-(−)-2-phenylglycinolAbsolute configuration: (2S,5S)
(2R,5S)-2-But-3-en-1-yl-5-phenylpyrrolidineC14H19N[α]D23=-48.9 (c 1.65, CHCl3)Source of chirality: (R)-(−)-2-phenylglycinolAbsolute configuration: (2R,5S)
(3S,5R,7aR)-3-Phenyl-5-[(phenylseleno)methyl]hexahydro-1H-pyrrolizineC20H23NSe[α]D30=-11.2 (c 1.05, CHCl3)Source of chirality: (R)-(−)-2-phenylglycinolAbsolute configuration: (3S,5R,7aR)
(3S,5R,7aS)-3-Phenyl-5-[(phenylseleno)methyl]hexahydro-1H-pyrrolizineC20H23NSe[α]D21=-16.7 (c 0.89, CHCl3)Source of chirality: (R)-(−)-2-phenylglycinolAbsolute configuration: (3S,5R,7aS)
(3S,5S,7aS)-3-Phenyl-5-[(phenylseleno)methyl]hexahydro-1H-pyrrolizineC20H23NSe[α]D18=-21.6 (c 0.68, CHCl3)Source of chirality: (R)-(−)-2-phenylglycinolAbsolute configuration: (3S,5S,7aS)
(5S,7aS)-5-[(Phenylseleno)methyl]hexahydro-3H-pyrrolizin-3-oneC14H17NOSe[α]D22=-83.6 (c 1.42, CHCl3)Source of chirality: (5R)-(−)-5-(hydroxymethyl)pyrrolidin-2-oneAbsolute configuration: (5S,7aS)
(5R,7aS)-5-[(Phenylseleno)methyl]hexahydro-3H-pyrrolizin-3-oneC14H17NOSe[α]D23=-6.9 (c 0.68, CHCl3)Source of chirality: (5R)-(−)-5-(hydroxymethyl)pyrrolidin-2-oneAbsolute configuration: (5R,7aS)
(5R,7aS)-5-Methylhexahydro-3H-pyrrolizin-3-oneC8H13NO[α]D23=-57.2 (c 2.49, CHCl3)Source of chirality: (5R)-(−)-5-(hydroxymethyl)pyrrolidin-2-oneAbsolute configuration: (5R,7aS)
(5S,7aS)-5-Methylhexahydro-3H-pyrrolizin-3-oneC8H13NO[α]D22=+4.6 (c 1.71, CHCl3)Source of chirality: (5R)-(−)-5-(hydroxymethyl)pyrrolidin-2-oneAbsolute configuration: (5S,7aS)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 20, 20 October 2008, Pages 2411–2416