| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345006 | 980169 | 2008 | 6 صفحه PDF | دانلود رایگان |
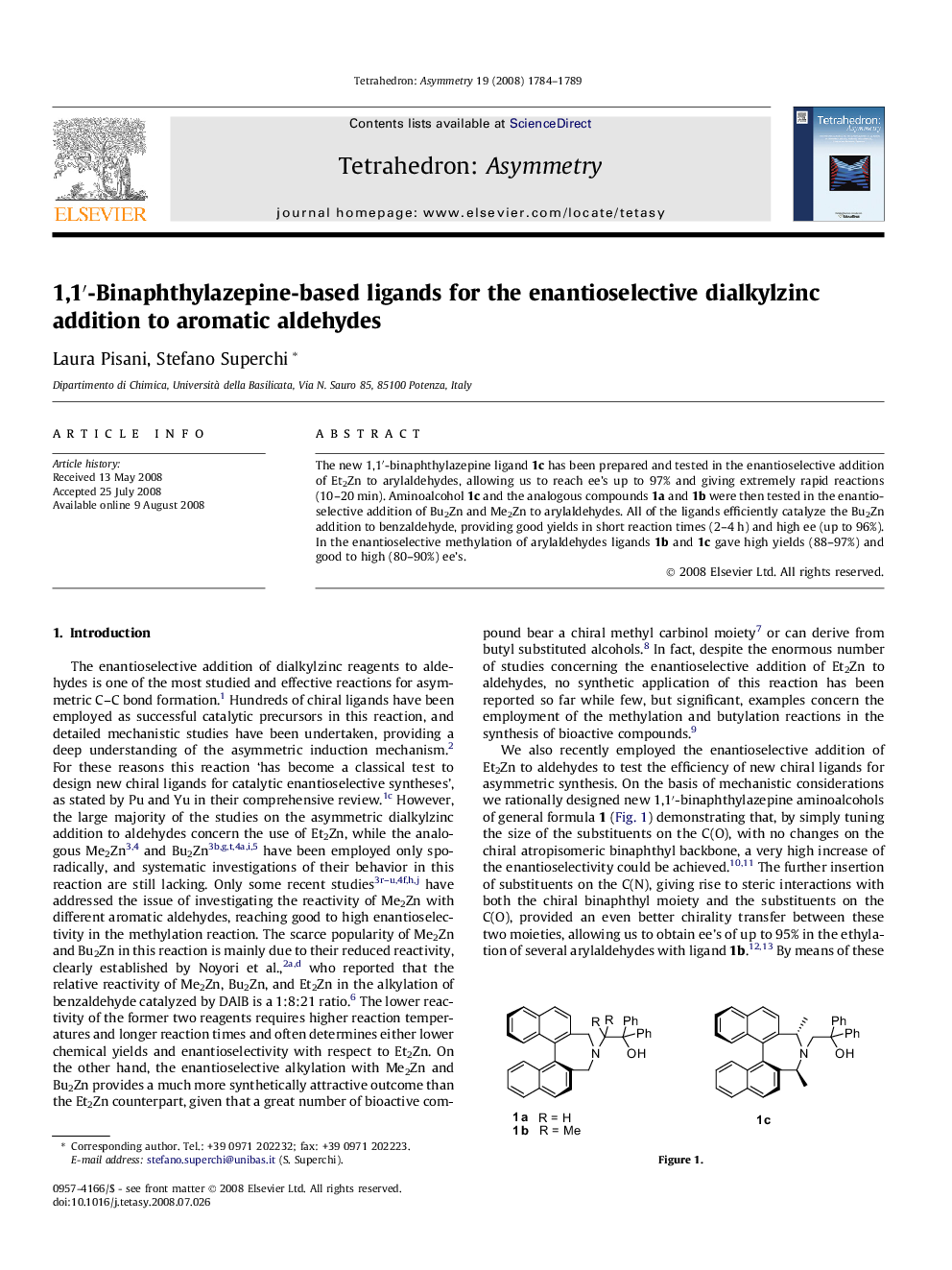
The new 1,1′-binaphthylazepine ligand 1c has been prepared and tested in the enantioselective addition of Et2Zn to arylaldehydes, allowing us to reach ee’s up to 97% and giving extremely rapid reactions (10–20 min). Aminoalcohol 1c and the analogous compounds 1a and 1b were then tested in the enantioselective addition of Bu2Zn and Me2Zn to arylaldehydes. All of the ligands efficiently catalyze the Bu2Zn addition to benzaldehyde, providing good yields in short reaction times (2–4 h) and high ee (up to 96%). In the enantioselective methylation of arylaldehydes ligands 1b and 1c gave high yields (88–97%) and good to high (80–90%) ee’s.
Figure optionsDownload as PowerPoint slide
(S)-(+)-3,5-Dihydro-4H-dinaphth[2,1-c:1′2′-e]azepine-N-hydroxideC22H17NOEe >99%[α]D21=+354.7 (c 1.02, CHCl3)Source of chirality: (S)-(−)-1,1′-bi-2-naphtholAbsolute configuration: (S)
(aS,S,S)-(+)-N-(2,2-Diphenyl-2-hydroxyethyl)-3,5-dihydro-3,5-dimethyl-4H-dinaphth[2,1-c:1′,2′-e]azepineC38H33NOEe >99%[α]D25=+3.2 (c 0.57, CHCl3)Source of chirality: (S)-(−)-1,1′-bi-2-naphtholAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 15, 8 August 2008, Pages 1784–1789