| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345009 | 980169 | 2008 | 6 صفحه PDF | دانلود رایگان |
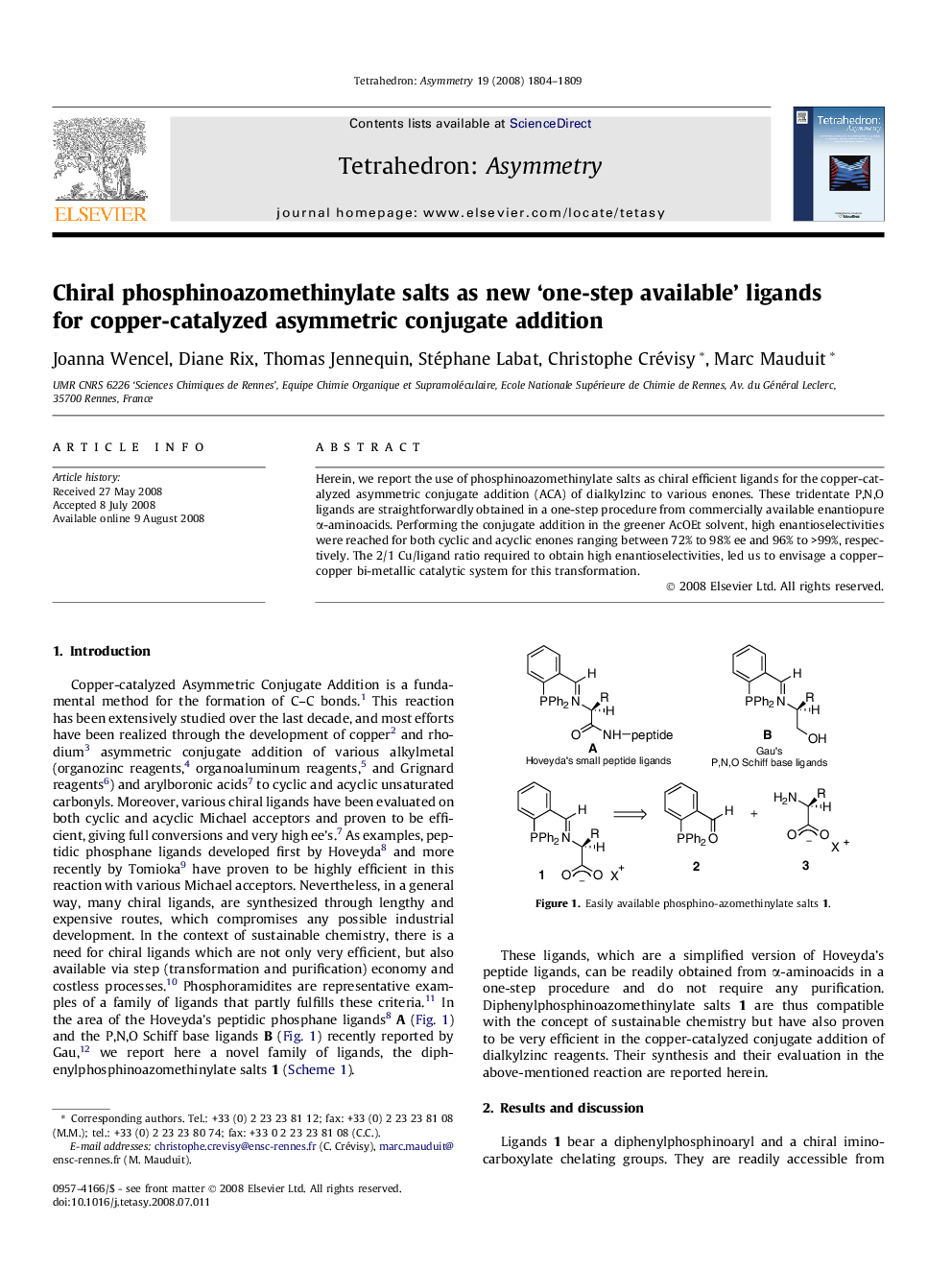
Herein, we report the use of phosphinoazomethinylate salts as chiral efficient ligands for the copper-catalyzed asymmetric conjugate addition (ACA) of dialkylzinc to various enones. These tridentate P,N,O ligands are straightforwardly obtained in a one-step procedure from commercially available enantiopure α-aminoacids. Performing the conjugate addition in the greener AcOEt solvent, high enantioselectivities were reached for both cyclic and acyclic enones ranging between 72% to 98% ee and 96% to >99%, respectively. The 2/1 Cu/ligand ratio required to obtain high enantioselectivities, led us to envisage a copper–copper bi-metallic catalytic system for this transformation.
Figure optionsDownload as PowerPoint slide
Sodium tert-leucine-diphenylphosphinoazomethinylateC25H26NNaO2P[α]20 = −11.4 (c 1, methanol)Source of chirality: (S)-tert-leucine 99% eeAbsolute configuration: (S)
Potassium tert-leucine-diphenylphosphinoazomethinylateC25H26NKO2P[α]20 = −11.0 (c 1, methanol)Source of chirality: (S)-tert-leucine 99% eeAbsolute configuration: (S)
Cesium tert-leucine-diphenylphosphinoazomethinylateC25H26NCsO2P[α]20 = −7.4 (c 1, methanol)Source of chirality: (S)-tert-leucine 99% eeAbsolute configuration: (S)
Lithium tert-leucine-diphenylphosphinoazomethinylateC25H26NLiO2P[α]20 = −11.5 (c 1, methanol)Source of chirality: (S)-tert-leucine 99% eeAbsolute configuration: (S)
Sodium valine-diphenylphosphinoazomethinylateC24H24NNaO2P[α]20 = −11.7 (c 1, methanol)Source of chirality: (S)-valine 99% eeAbsolute configuration: (S)
Sodium iso-leucine-diphenylphosphinoazomethinylateC25H26NNaO2P[α]20 = −15.0 (c 1, methanol)Source of chirality: (S,S)-iso-leucine 99% eeAbsolute configuration: (S,S)
Sodium leucine-diphenylphosphinoazomethinylateC25H26NNaO2P[α]20 = −12.3(c 1, methanol)Source of chirality: (S)-valine 99% eeAbsolute configuration: (S)
Sodium phenylglycine-diphenylphosphinoazomethinylateC27H22NNaO2P[α]20 = +4.5 (c 1, methanol)Source of chirality: (S)-phenylglycine 99% eeAbsolute configuration: (S)
Valine-diphenylphosphinoazomethinylate methyl esterC25H26NO2P[α]20 = −9.1 (c 1, methanol)Source of chirality: (S)-leucine 99% eeAbsolute configuration: (S)
Sodium tert-leucine-azomethinylateC13H17NNaO2[α]20 = −3.2 (c 1, methanol)Source of chirality: (S)-tert-leucine 99% eeAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 15, 8 August 2008, Pages 1804–1809