| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345050 | 980173 | 2014 | 6 صفحه PDF | دانلود رایگان |
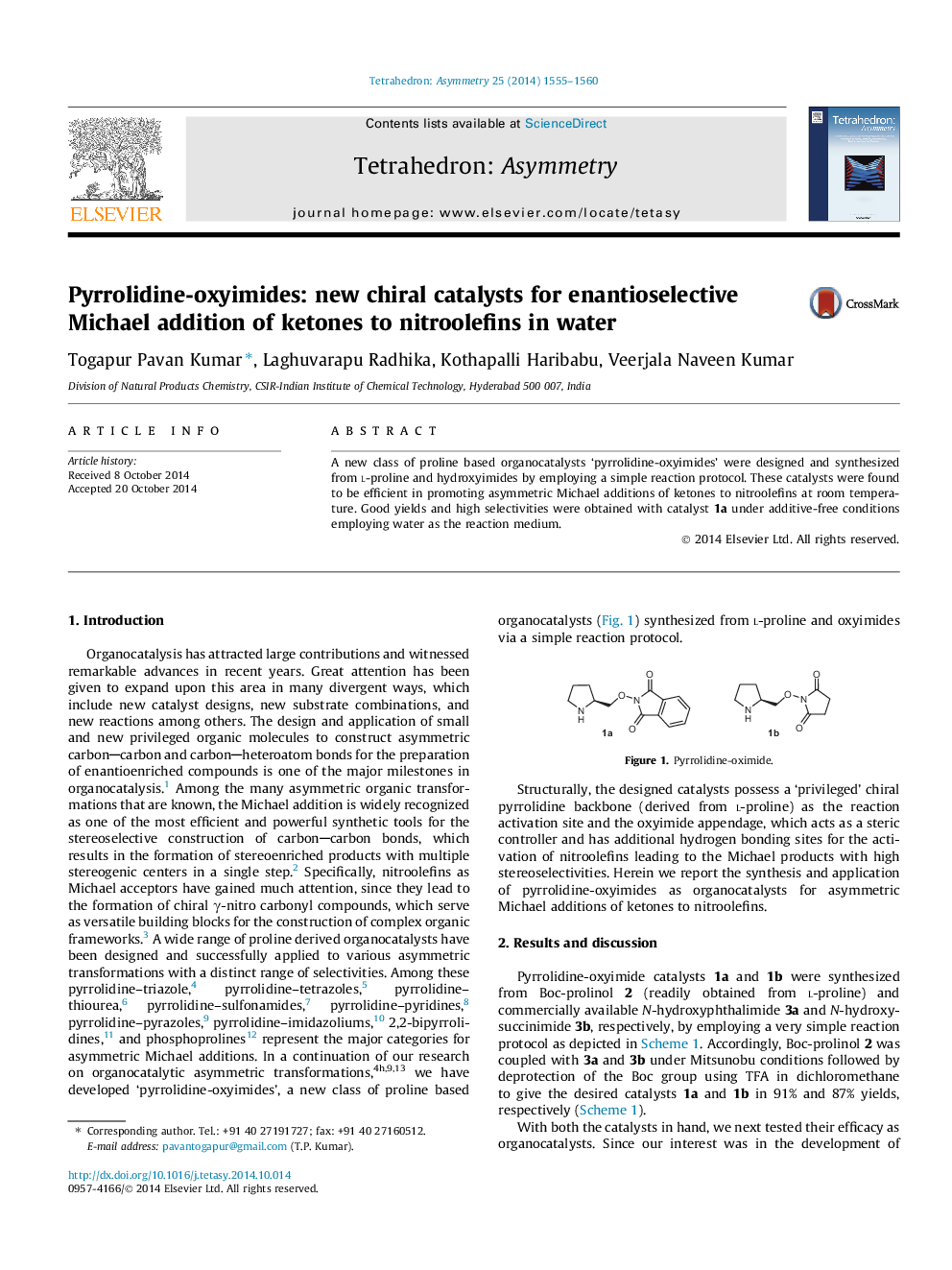
A new class of proline based organocatalysts ‘pyrrolidine-oxyimides’ were designed and synthesized from l-proline and hydroxyimides by employing a simple reaction protocol. These catalysts were found to be efficient in promoting asymmetric Michael additions of ketones to nitroolefins at room temperature. Good yields and high selectivities were obtained with catalyst 1a under additive-free conditions employing water as the reaction medium.
Figure optionsDownload as PowerPoint slide
(S)-tert-Butyl-2-((1,3-dioxoisoindolin-2-yloxy)methyl)pyrrolidine-1-carboxylateC18H22N2O5[α]D25 = −49.3 (c 2, CHCl3)Source of chirality: (S)-ProlineAbsolute configuration: (S)
(S)-2-(Pyrrolidin-2-ylmethoxy)isoindoline-1,3-dioneC13H14N2O3[α]D25 = +15.2 (c 3, CHCl3)Source of chirality: (S)-ProlineAbsolute configuration: (S)
(S)-tert-Butyl-2-((2,5-dioxopyrrolidin-1-yloxy)methyl)pyrrolidine-1-carboxylateC14H22N2O5[α]D25 = −54.5 (c 1, CHCl3)Source of chirality: (S)-ProlineAbsolute configuration: (S)
(S)-1-(Pyrrolidin-2-ylmethoxy)pyrrolidine-2,5-dioneC9H14N2O3[α]D25 = + 50 (c 1, MeOH)Source of chirality: (S)-ProlineAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 25, Issue 23, 15 December 2014, Pages 1555–1560