| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345059 | 980174 | 2008 | 4 صفحه PDF | دانلود رایگان |
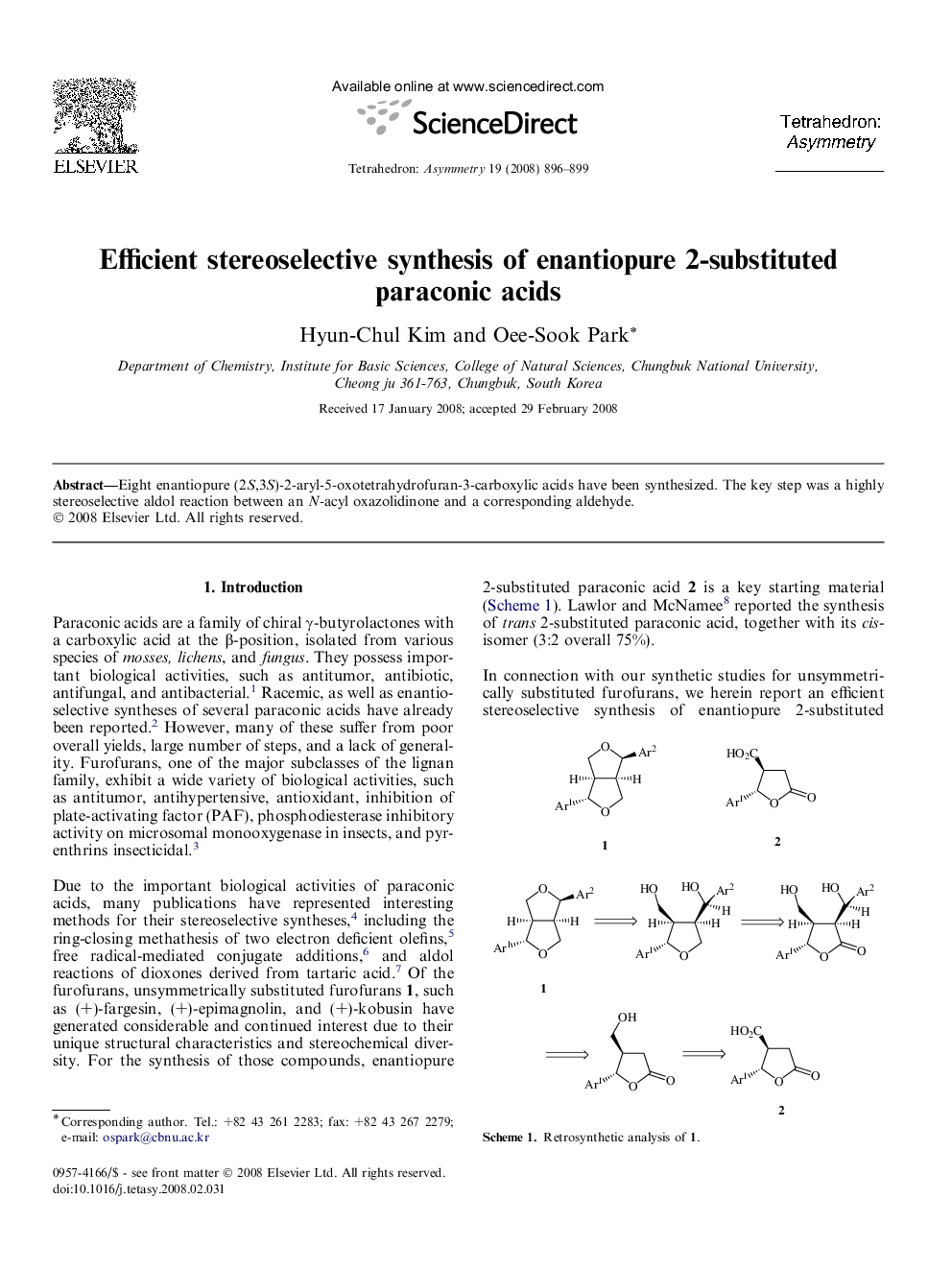
Eight enantiopure (2S,3S)-2-aryl-5-oxotetrahydrofuran-3-carboxylic acids have been synthesized. The key step was a highly stereoselective aldol reaction between an N-acyl oxazolidinone and a corresponding aldehyde.
Figure optionsDownload as PowerPoint slide
(2S,3S)-2-(Benzo[d][1,3]dioxol-5-yl)tetrahydro-5-oxofuran-3-carboxylic acidC12H10O6[α]D25=−44.2 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3S)
(2S,3S)-Tetrahydro-5-oxo-2-phenylfuran-3-carboxylic acidC11H10O4[α]D25=−37.4 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3S)
(2S,3S)-Tetrahydro-5-oxo-2-p-tolylfuran-3-carboxylic acidC12H12O4[α]D25=−31.3 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 8, 1 May 2008, Pages 896–899