| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345073 | 980174 | 2008 | 7 صفحه PDF | دانلود رایگان |
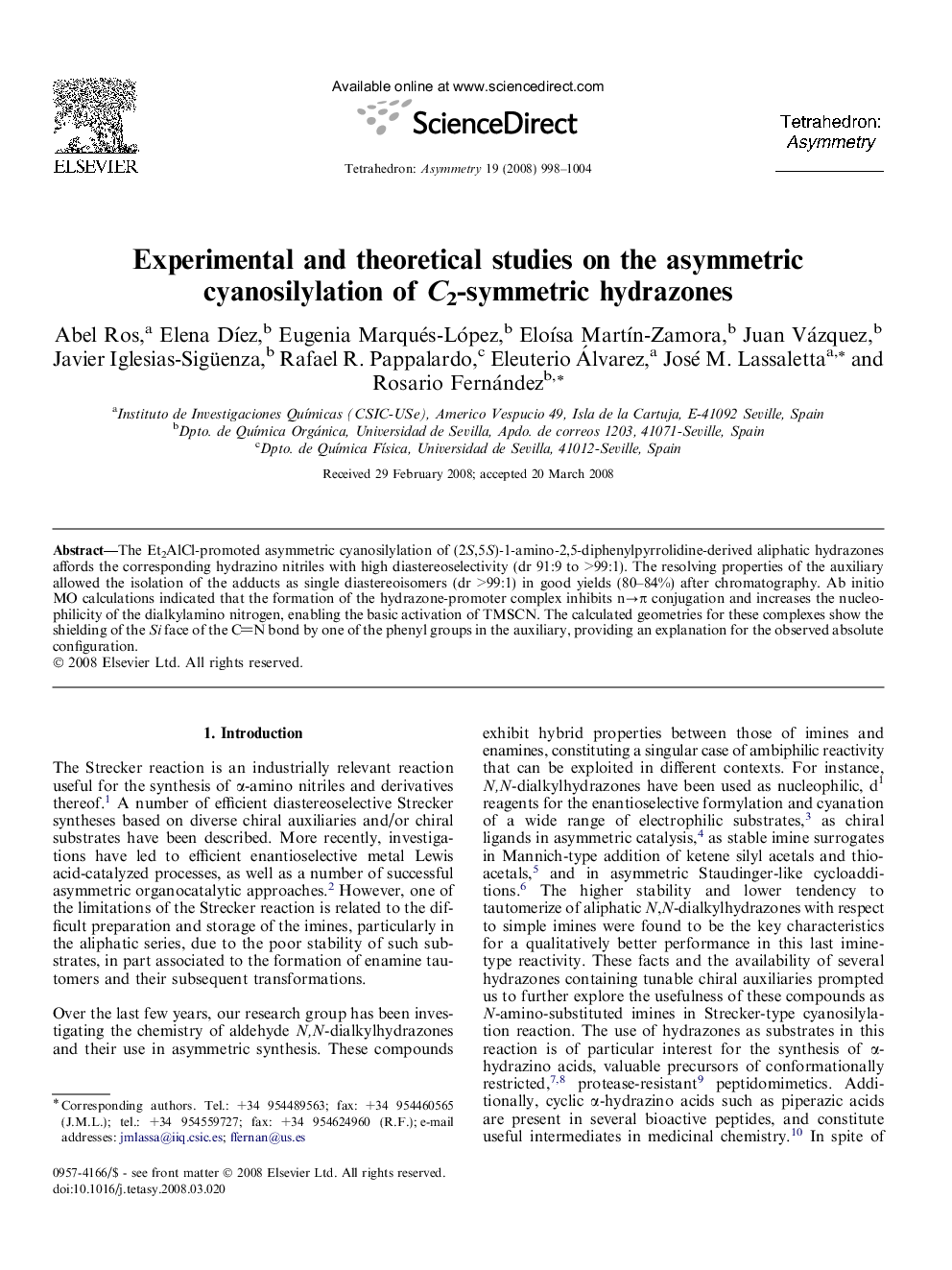
The Et2AlCl-promoted asymmetric cyanosilylation of (2S,5S)-1-amino-2,5-diphenylpyrrolidine-derived aliphatic hydrazones affords the corresponding hydrazinonitriles with high diastereoselectivity (dr 91:9 to >99:1). The resolving properties of the auxiliary allowed the isolation of the adducts as single diastereoisomers (dr >99:1) in good yields (80–84%) after chromatography. Ab initio MO calculations indicated that the formation of the hydrazone-promoter complex inhibits n→π conjugation and increases the nucleophilicity of the dialkylamino nitrogen, enabling the basic activation of TMSCN. The calculated geometries for these complexes show the shielding of the Si face of the CN bond by one of the phenyl groups in the auxiliary, providing an explanation for the observed absolute configuration.
Figure optionsDownload as PowerPoint slide
(2S,5S)-1-[(3-Phenyl)propylideneamine]-2,5-diphenylpyrrolidineC25H26N2Ee = 100%[α]D20=-163.9 (c 1.4, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5S)
(2S,5S)-1-[(2-Methyl)propylideneamine]-2,5-diphenylpyrrolidineC20H24N2Ee = 100%[α]D20=-149.7 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5S)
(2S,5S)-1-Hexylideneamine-2,5-diphenylpyrrolidineC22H28N2Ee = 100%[α]D20=-149.0 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5S)
(R)-2-[(2S,5S)-2,5-Diphenylpyrrolidin-1-ylamino]-4-phenylbutanenitrileC26H27N3Ee = 100%[α]D20=-138.0 (c 1.0, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: (R)(2S,5S)
(R)-2-[(2S,5S)-2,5-Diphenylpyrrolidin-1-ylamino]-3-methylbutanenitrileC21H25N3Ee = 100%[α]D20=-125.3 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)(2S,5S)
(R)-2-[(2S,5S)-2,5-Diphenylpyrrolidin-1-ylamino]-4-methylpentanenitrileC22H27N3Ee = 100%[α]D20=-142.8 (c 1.5, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)(2S,5S)
(R)-2-[(2S,5S)-2,5-Diphenylpyrrolidin-1-ylamino]heptanenitrileC23H29N3Ee = 100%[α]D20=-152.3 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)(2S,5S)
(R)-2-[(2S,5S)-2,5-Diphenylpyrrolidin-1-ylamino]-4-methylpentanenitrile hydrochlorideC22H28ClN3Ee = 100%[α]D20=-106.1 (c 1.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)(2S,5S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 8, 1 May 2008, Pages 998–1004