| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345092 | 1500345 | 2014 | 6 صفحه PDF | دانلود رایگان |
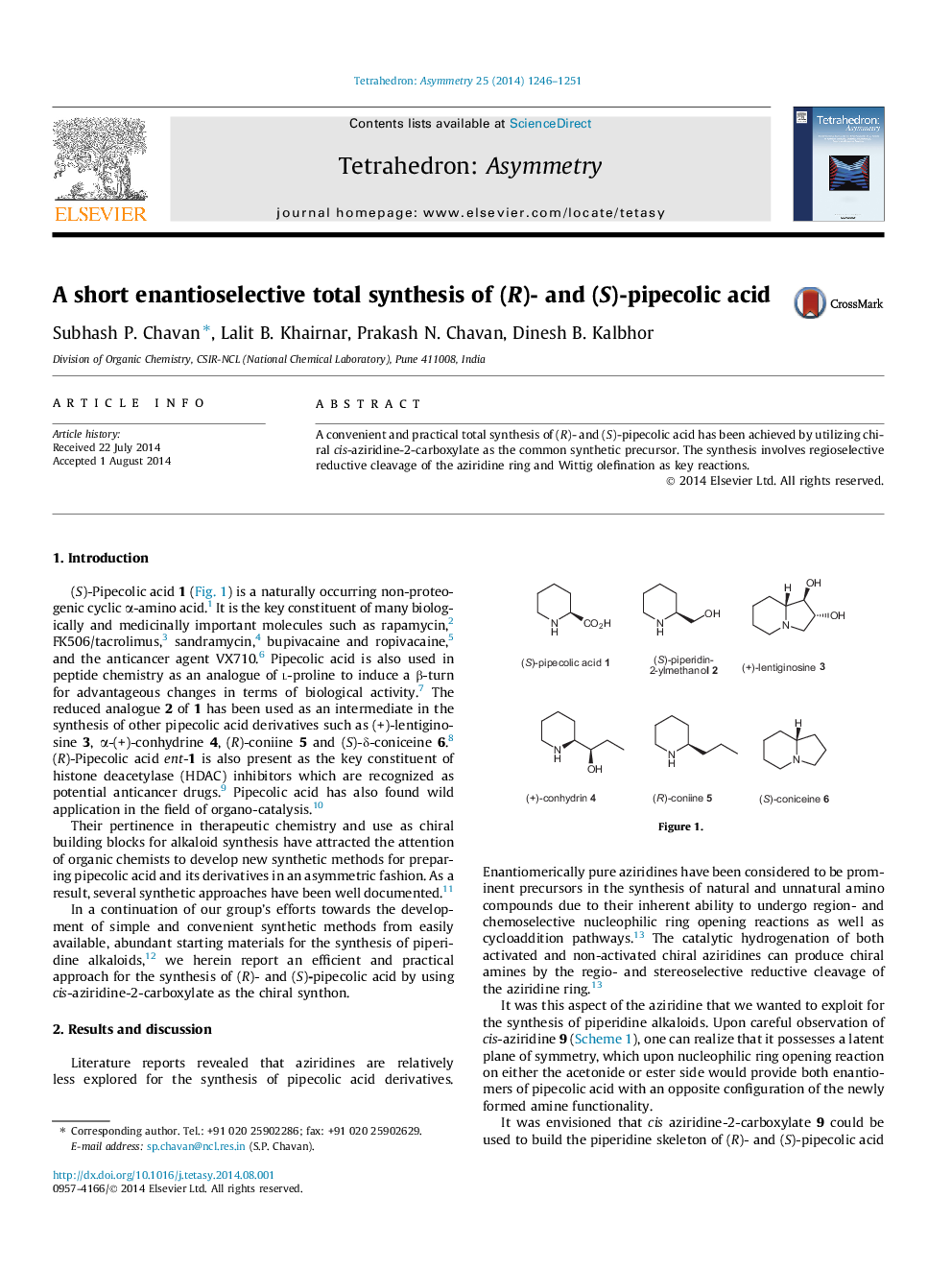
A convenient and practical total synthesis of (R)- and (S)-pipecolic acid has been achieved by utilizing chiral cis-aziridine-2-carboxylate as the common synthetic precursor. The synthesis involves regioselective reductive cleavage of the aziridine ring and Wittig olefination as key reactions.
Figure optionsDownload as PowerPoint slide
(E)-Ethyl-3-((2R,3R)-1-benzyl-3-(2,2-dimethyl-1,3-dioxolan-4-yl)aziridin-2-yl)acrylateC19H25NO4[α]D25 = +29.4 (c 1.7, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (2R,3R)
(R)-6-((S)-2,2-Dimethyl-1,3-dioxolan-4-yl)piperidin-2-oneC10H17NO3[α]D25 = −20.0 (c 1.1, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (R)(S)
(R)-Benzyl 2-((S)-1,2-dihydroxyethyl)piperidine-1-carboxylateC15H21NO4[α]D25 = +35.4 (c 1, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (R)(S)
(R)-1-((Benzyloxy)carbonyl)piperidine-2-carboxylic acidC14H17NO4[α]D25 = +55.1 (c 1.1, AcOH)Source of chirality: d-MannitolAbsolute configuration: (R)
(R)-Piperidine-2-carboxylic acidC6H11NO2[α]D25 = +24.9 (c 1.15, H2O)Source of chirality: d-MannitolAbsolute configuration: (R)
((2S,3R)-1-Benzyl-3-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)aziridin-2-yl)methanolC15H21NO3[α]D25 = +32.1 (c 0.85, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (2S,3R)(S)
(2S,3R)-1-Benzyl-2-((benzyloxy)methyl)-3-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)aziridineC22H27NO3[α]D25 = −23 (c 1.2, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (2S,3R)(S)
(S)-1-((2R,3S)-1-Benzyl-3-((benzyloxy)methyl)aziridin-2-yl)ethane-1,2-diolC19H23NO3[α]D25 = −20 (c 1, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (S)(2R,3S)
(E)-Ethyl 3-((2S,3S)-1-benzyl-3-((benzyloxy)methyl)aziridin-2-yl)acrylateC22H25NO3[α]D25 = −48.5 (c 1.03, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (2S,3S)
(S)-6-((Benzyloxy)methyl)piperidin-2-oneC13H17NO2[α]D25 = +10.1 (c 1.19, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (S)
(S)-tert-Butyl 2-(hydroxymethyl)piperidine-1-carboxylateC11H21NO3[α]D25 = −38.2 (c 1.2, CHCl3)Source of chirality: d-MannitolAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 16–17, 15 September 2014, Pages 1246–1251