| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345114 | 980179 | 2008 | 16 صفحه PDF | دانلود رایگان |
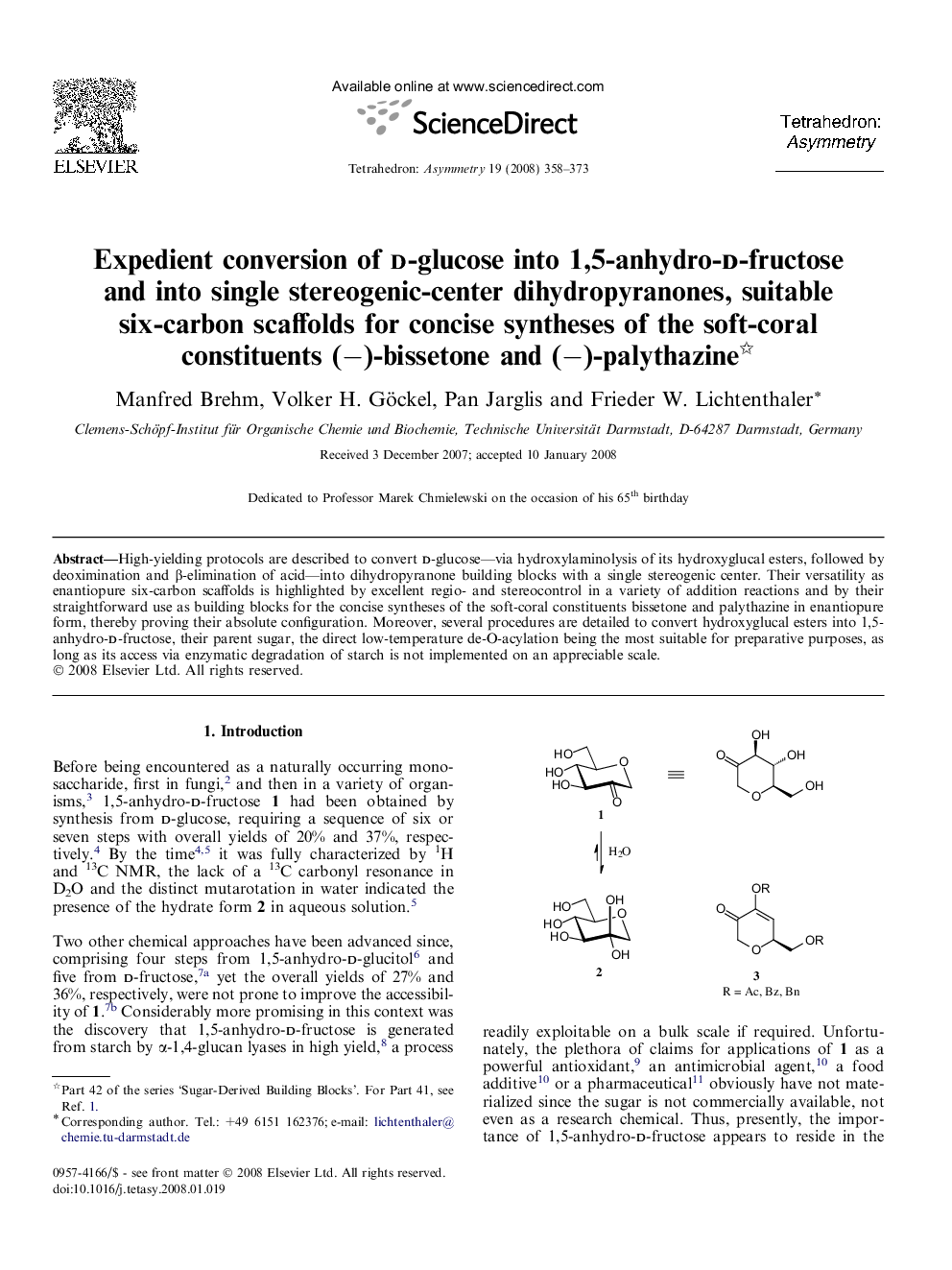
High-yielding protocols are described to convert d-glucose—via hydroxylaminolysis of its hydroxyglucal esters, followed by deoximination and β-elimination of acid—into dihydropyranone building blocks with a single stereogenic center. Their versatility as enantiopure six-carbon scaffolds is highlighted by excellent regio- and stereocontrol in a variety of addition reactions and by their straightforward use as building blocks for the concise syntheses of the soft-coral constituents bissetone and palythazine in enantiopure form, thereby proving their absolute configuration. Moreover, several procedures are detailed to convert hydroxyglucal esters into 1,5-anhydro-d-fructose, their parent sugar, the direct low-temperature de-O-acylation being the most suitable for preparative purposes, as long as its access via enzymatic degradation of starch is not implemented on an appreciable scale.
Figure optionsDownload as PowerPoint slide
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose semicarbazoneC13H19N3O8[α]D20=-72.5 (c 0.2, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-benzyloximeC19H23NO8[α]D21=-39 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose E-oximeC12H17NO8[α]D20=-42.8 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructose E-oximeC27H23NO8[α]D22=-52.9 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzyl-1,5-anhydro-d-fructose E-oximeC27H29NO5[α]D20=-29.0 (c 1.1, CHCl3)Source of chirality: d-glucose
1,5-Anhydro-d-fructose E-oximeC6H11NO5[α]D21=-43.0 (c 0.3, water)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-methyloximeC13H19NO8[α]D21=-29 (c 1, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-acetyloximeC14H19NO9[α]D21=-49 (c 0.3, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-methanesulfonyloximeC13H19NO10S[α]D22=-56.6 (c 0.3, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructoseC12H16O8[α]D21=-10 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructoseC27H22O8[α]D20=-29.2 (c 0.6, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzyl-1,5-anhydro-d-fructopyranoseC27H28O5[α]D20=-16.1 (c 1.1, CHCl3)Source of chirality: d-glucose
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-oneC20H16O6[α]D20=-16.0 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzyloxy-6-benzyloxymethyl-2H-pyran-3(6H)-oneC20H20O4[α]D20=-32.1 (c 1.1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructose diethyldithioacetalC31H32O7S2[α]D23=-51 (c 1.1, CHCl3)Source of chirality: d-glucose
1,5-Anhydro-d-fructose diethyldithioacetalC10H20O4S2[α]D24=-49.8 (c 1, CHCl3)Source of chirality: d-glucose
(6S)-6-Benzoyloxymethyl-3,3-dimethoxy-tetrahydropyran-4-oneC15H18O6[α]D20=-85.6 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzoyloxymethyl-3,3-di(benzyloxy)-tetrahydropyran-4-oneC27H26O6[α]D = −94.8 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-one oximeC20H17NO6[α]D21=-22 (c 0.6, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-one phenylhydrazoneC26H22N2O5[α]D20=-14 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzyloxymethyl-tetrahydropyran-3,4-dione dioximeC13H14N2O5[α]D21=-111 (c 0.5, pyridine)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzyloxymethyl-tetrahydropyran-3,4-dione bis(phenylhydrazone)C25H24N4O3[α]D20=-114.3 (c 1.0, CHCl3)[α]D20=-64.3 (c 1.0, pyridine)Source of chirality: d-glucoseAbsolute configuration: (6S)
(3S)-3-Benzyloxymethyl-3,4-dihydro-1H-pyrano[3,4-b]quinoxalineC19H16N2O3[α]D = −81.3 (c 1.1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (3S)
(2S,4S,5R)-4-Benzoyloxy-2-benzoyloxymethyl-5-hydroxy-tetrahydropyranC20H20O6[α]D25=-18.6 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,4S,5R)
3,6-Di-O-benzoyl-4-deoxy-4-C-methyl-1,5-anhydro-d-fructoseC21H22O7[α]D20=-6.3 (c 0.5, CHCl3)Source of chirality: d-glucose
(2S,5S)-5-Benzoyloxy-2-benzoyloxymethyl-5-methyl-tetrahydropyran-4-oneC21H20O6[α]D20=-89 (c 1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
(2S,5S)-5-Allyl-5-benzoyloxy-2-benzoyloxymethyl-tetrahydropyran-4-oneC23H22O6[α]D20=-65 (c 0.7, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,6S)
(2S)-2-Benzoyloxymethyl-5,5-bis(nitromethyl)-tetrahydropyran-4-oneC15H16N2O8[α]D20=-34.2 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S)
(2S,5S)-2-Benzoyloxymethyl-5-hydroxy-5-acetonyl-tetrahydropyran-4-oneC16H18O6[α]D20=-31.6 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
(−)-Bissetone[(2S,5S)-5-acetonyl-5-hydroxy-2-hydroxymethyl-tetrahydropyran-4-one]C9H14O5[α]D20=-69.4 (c 1.2, EtOH)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
S,S-Palythazine [(3S,8S)-1,3,4,6,8,9-hexahydro-dipyrano[3,4-b:3′,4′-e]pyrazine-3,8-dimethanol]C12H16N2O4[α]D = −198.8 (c 0.35, CH3OH)Source of chirality: d-glucoseAbsolute configuration: (3S,8S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 3, 19 February 2008, Pages 358–373